Ursodeoxycholic acid (UDCA) is the first choice medication for most cholestatic hepatopathies, due to its capability to counteract inflammation and bile-acid-induced liver damage, two common features in cholestasis. However, UDCA is usually contraindicated in obstructive cholestasis, due to the alleged risk of biliary integrity disruption due to its choleretic effect. We report on an 83-year-old man with an unsuspected malignant biliary obstruction who received moderate doses of UDCA (8-12 mg/kg/day) for 5 weeks, because the preliminary evidence suggested he had chemotherapy-induced cholestasis. Liver integrity was extensively protected by UDCA, as indicated by a marked decrease in serum liver enzymes, despite a steady increase in the levels of bilirubin and serum bile acids due to the obstructive process. In conclusion, this report shows, for the first time in humans, that moderate UDCA doses can reduce liver injury associated with complete biliary obstruction. This may contribute to a better understanding of the risk-benefit ratio of the use of UDCA in obstructive cholangiopathies.
Ursodeoxycholic acid (UDCA) is a therapeutic agent widely used for the treatment of cholestatic hepatopathies of diverse etiology. Such versatility is accounted for by its multiple mechanisms of action, including:1
- •
Protection against bile acid-induced necrotic and apoptotic cell death.
- •
Induction of metabolic changes that reduces bile acid levels and toxicity.
- •
Protection of cholangiocytes by choleresis-induced dilution of luminal bile acid, and restoration of protective levels of biliary phospholipids and bicarbonate often impaired in cholangiopathies.
- •
Anti-inflammatory and immunomodulatory properties that limit the exacerbated immunological response and liver fibrosis occurring in cholestatic diseases, among others.
All these cytoprotective mechanisms are expected to be of great benefit to preserve liver integrity in obstructive cholestasis, where cytotoxic bile acids built up at the highest levels due to the total biliary blockage, and where an overt proinflammatory/profibrotic response takes place rapidly. However, the common view among hepatologists is that UDCA has to be administrated with extreme caution, or even contraindicated, in patients suspected to have cholangiopathies with a predominant obstructive component, e.g., late stages of primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), or biliary atresia. Furthermore, deleterious effects of UDCA has been argued to explain in part the limited therapeutic efficiency, or even the detrimental effects of UDCA in late-stage PBC2 and PSC.3 Similarly, UDCA therapeutic use in biliary atresia has been restricted to the type-III one (ductules > 50 μm).4
In this article, we report on a case study of a patient with an unsuspected malignant biliary obstruction who received moderate doses of UDCA for 5 weeks, because the initial clinical and radiological evidence suggested that he had chemotherapy-induced intrahepatic cholestasis. Surprisingly, hepatic integrity was significantly improved by UDCA, as indicated by the sustained decrease in the release into the bloodstream of liver enzymes during the whole treatment period, despite the ongoing biliary obstruction.
Case ReportThe patient, an 83-year-old man, had undergone a left colectomy in 2004 for a stage I colon adenocarcinoma, and, in August 2007, a partial hepatectomy for removal of two metastatic nodules, followed by 6 months of chemotherapy. He remained tumor-free until May 2010, when two pulmonary nodules were identified and resected. In September 2013, recurrence of multiple pulmonary micronodules occurred, and chemotherapy with the chimeric IgG1 monoclonal antibody cetuximab, an epithelial growth factor receptor (EGFR) inhibitor, was prescribed as monotherapy. After 3 months, chemotherapy was interrupted, because the patient had developed an intense acneiform eruption, a common adverse effect of therapy with EGFR inhibitors.
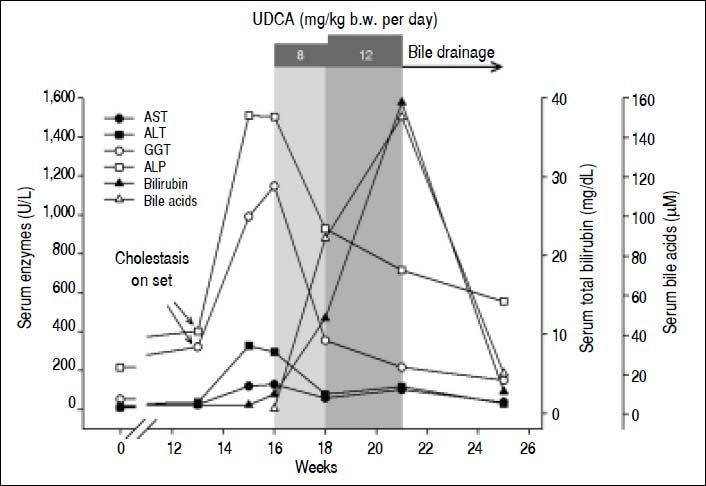
A computed tomography (CT) scan performed immediately after chemotherapy interruption demonstrated that most lung nodules remained unchanged. Additionally, there was no evidence of liver metastases or metastatic abdominal lymphadenopathies, and bile ducts were not dilated. Surprisingly, simultaneous liver tests (LTs) revealed a mild increase in the cholestatic enzymes, gamma glutamyl transpeptidase (GGT) and alkaline phosphatase (ALP), with normal aminotransferase and total-bilirubin serum values (Figure 1).
Time course of the serum levels of AST, ALT, GGT, ALP, bilirubin and bile acids throughout the follow-up period. Onset of cholestasis was detected early, as a mild, isolated increment in the cholestatic enzymes GGT and ALP, as compared with the normal values recorded 3 month before. The serum levels of these enzymes increased sharply during the next 2 weeks, and were accompanied with increments in serum aminotransferase levels, and a mild elevation of serum tota bilirubin levels. With a presumptive diagnosis of intrahepatic cholestasis induced by a recent chemotherapy in mind, UDCA was prescribed (shadow area). At the dose of 8 mg/kg/day for 2 weeks, UDCA reduced the serum levels of cholestatic enzymes and normalized aminotransferase levels, suggesting protection against bile acid-induced hepato- and cholangiocellular damage. In contrast, serum levels of both total bilirubin and bile acids started to rise quickly. The serum levels of cholestatic enzymes were reduced further when the UDCA dose was increased to 12 mg/kg/day for a further 3-week period, but serum levels of both total bilirubin and bile acid continued to raise significantly. At that moment, the patient was re-examined for biliary obstruction by retrograde cholangiography, and an extrinsic compression of the bile ducts was identified. A biliary stent was placed to relieve the obstructions, which was followed by a rapid drop of both bilirubin and bile acid levels.
A repeat set of LTs two weeks later showed worsening of the biochemical cholestatic pattern, plus an increase in serum aminotransferases (Figure 1). One week later, an increase in total serum bilirubin levels (x 2 ULN) was seen, with direct (conjugated) bilirubin being predominant (93%), and with normal serum bile acid values. Autoimmune and viral liver diseases were ruled out by appropriate serological tests.
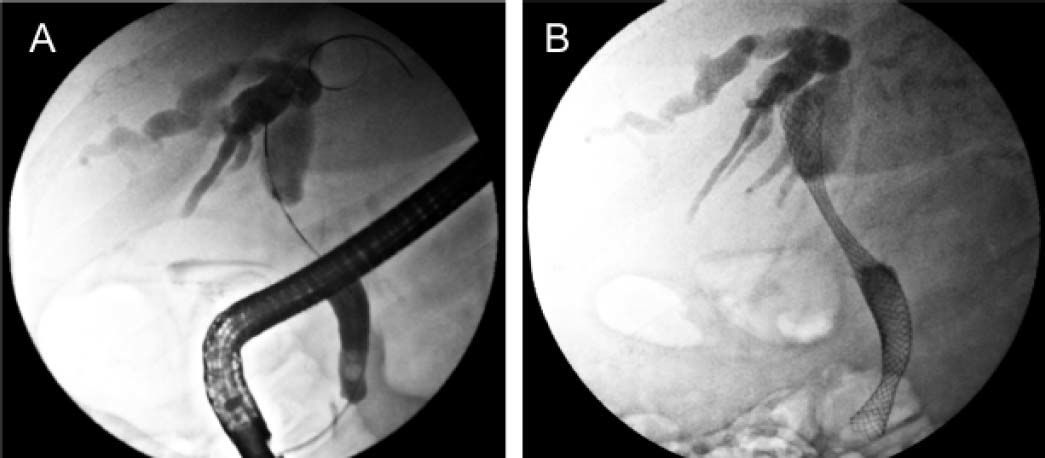
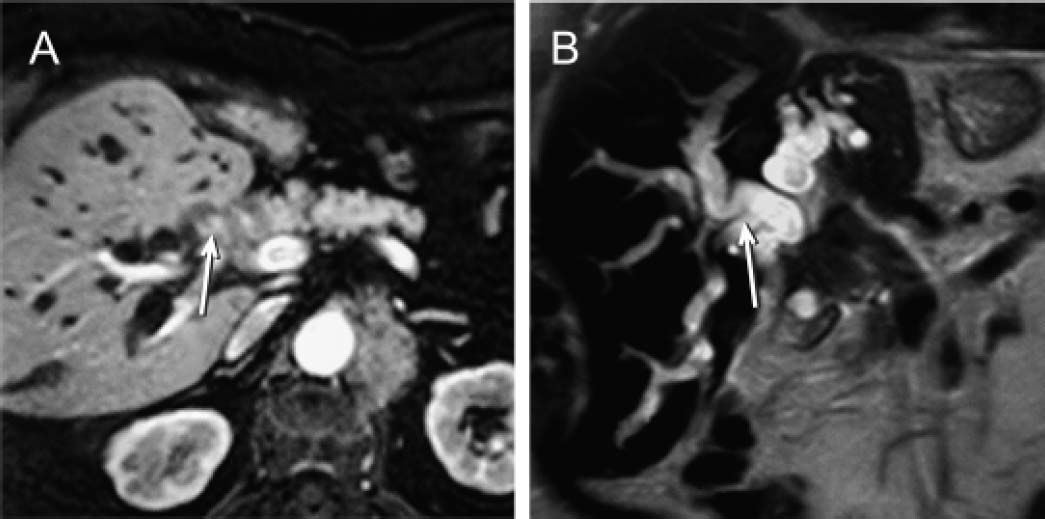
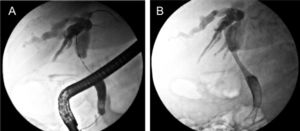
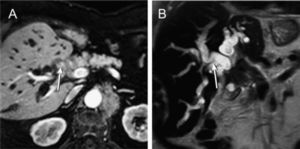
From the clinical point of view, moderate pruritus and digestive symptoms were reported by the patient. Based on a presumed diagnosis of intrahepatic cholestasis associated with cetuximab chemotherapy, we prescribed UDCA (8 mg/kg/day; 300 mg/capsule, 2 capsules per day). After 2 weeks of UDCA treatment, the patient was itch free, GGT and ALP values were 2.8 and 1.6 times lower than the pretreatment values, respectively, and serum aminotransferase activities were normal (Figure 1). In contrast, a 5-fold increase in total serum bilirubin levels was recorded, with predominance of conjugated bilirubin (83%), and serum bile acid values increased sharply to 90 μM (x 9 ULN). We interpreted these paradoxical increments as a result of enhanced hepatocellular extrusion of both bilirubin and bile acids into blood due to induction of basolateral export pumps, a well recognized anticholestatic mechanism of UDCA.1 Therefore, we decided to increase UDCA dose from 8 to 12 mg/kg/day (300 mg/capsule, 3 capsules per day). Subsequent LTs carried out 3 weeks later showed a further decrease in the serum activities of GGT (− 39%) and ALP (− 23%). On the contrary, serum levels of both bile acids and total (80% conjugated) bilirubin increased further (+69% and +226%, respectively). This dissociated pattern, with decreased liver enzyme serum activities but increased conjugated bilirubin and bile acid ones was more compatible with an ongoing biliary obstruction whose deleterious effects on liver had been efficiently counteracted by UDCA. UDCA therapy was then stopped, and the patient re-examined for biliary obstruction by ultrasonography. This study revealed dilated intrahepatic and extrahepatic biliary tract. The nature and exact location of the obstruction was demonstrated by endo-scopic retrograde cholangiopancreatography (Figure 2A). An additional magnetic resonance cholangiopancreatography scan showed that the biliary obstruction was due to an ill-defined mass located at the distal common hepatic duct and proximal common bile duct, likely from metastatic lymphadenopathy or infiltration of the peribiliary tissues (Figure 3).
Pre- and post-biliary stent placement for palliation of the malignant biliary obstruction. A. Endoscopic retrograde cholangiopancreatography reveals extrinsic compression of the dista common hepatic duct and proximal bile common duct (arrow), accompanied with intrahepatic biliary dilation, due to metastases in nearby lymph nodes or peribiliary tissue. B. An uncovered, self-expandable metal stents was implanted in the obstructed ducts to relieve the blockage.
After obtaining the patient’s oral consent, a biliary stent was placed by endoscopic retrograde cholangiopancreatography for relief of the biliary obstruction (Figure 2B). One month after stent placement, liver tests had significantly improved (Figure 1). The patient remained clinically stable after 4 months of follow-up, post biliary-stent placement.
DiscussionCholestasis is not an unusual feature in advanced colon cancer. It is often produced by liver parenchymal metastasis, but it can be more rarely caused by extrinsic compression of the extrahepatic bile ducts due to either metastasis to the perihilar lymph nodes or tumor invasion to the peribiliary connective tissue,5 as in our case.
Metastatic biliary obstruction can develop very fast, and biochemical signs of cholestasis can precede the diagnosis by abdominal imaging, as illustrated by this case. Indeed, a mild, isolated increase in ALP and GGT was recorded in our patient without CT evidence of any mass extrinsically compressing bile ducts; incidentally, this points to the high sensitivity of these cholestasis enzymes in identifying early obstruction, as compared with other liver tests, such as serum bilirubin and bile acids, which were normal at that moment. Two weeks later, complete obstruction seems to have developed, since ALP and GGT serum values (x 11 and x 18 ULN, respectively) were compatible with those reported in fully obstructed patients.6 ALP is a membrane-bound enzyme from cholangiocytes and hepatocytes that is further induced and released into the bloodstream by detergent bile acids that accumulate in cholestasis,7 whereas serum elevations of GGT, another membrane-bound enzyme, are thought to be exclusively due to bile acid-induced release from cholangiocytes.8 Therefore, ALP/GGT increments in blood reflect hepatic bile acid accumulation with the consequent impairment of integrity of the liver cell membranes due to the detergent effect of endogenous bile acids, leading to cell death. The delayed increase in serum conjugated bilirubin and bile acids was, in turn, more likely associated with biliary secretory failure of the pigment due to the obstructive process rather than to severe liver damage, since serum transaminase activity, a surrogate parameter of hepatocellular membrane integrity and necrosis, remained modestly elevated at the same point in time.
Lack of initial CT evidence of biliary obstruction and recent adverse effects of chemotherapy were misleading factors that led us to a presumptive diagnosis of drug-induced intrahepatic cholestasis. Actually, intrahepatic cholestasis is a rare but increasingly acknowledged side effect of IgG1 monoclonal antibody therapy.9–12
With the diagnosis of drug-induced liver injury in mind, UDCA was prescribed for several reasons. First, the patient reported increasing pruritus, and UDCA effectively attenuates itching in several kinds of intrahepatic cholestasis.10 Second, UDCA was expected to aid in preserving both hepatocyte and cholangiocyte integrity and function in the face of accumulating toxic, endogenous bile acid levels, while waiting for the spontaneous recovery after cetuximab withdrawal. At the therapeutic doses employed here, UDCA administration was shown to decrease hydrophobicity of the bile acid pool by displacing/ replacing toxic (hydrophobic), endogenous bile acids;1 because of the low UDCA hydrophobicity, this is associated with far less membrane protein removal. The significant dose-dependent reduction in serum activities of ALP and GGT seen with this patient during UDCA treatment seemed therefore to validate our therapeutic strategy.
Having an obstructive rather than an hepatocellular form of cholestasis been confirmed afterwards, different and perhaps more relevant conclusions can be drawn from this case. Overall, our clinical case challenges the general view that UDCA has always detrimental effects in patients with obstructive cholestasis. Our data rather show that, on the contrary, UDCA can even be helpful, both in terms of symptoms and liver biochemistry, at least at the moderate UDCA dosage and the short treatment period employed here.
It is difficult to ascertain why the concept that UDCA is highly detrimental in cholangiopathies with biliary obstruction is so tightly rooted in the clinical practice. Part of this concern was supported by experiments in mice showing that UDCA feeding to both common-bile-duct-ligated (CBDL) mice and Mdr2(-/-) mice (a PSC animal model) induces biliary infarcts, leading to leakage of bile into the parenchyma via disrupted Herring’s canals, and further hepatocyte cell death.13 In addition, the potential toxicity of UDCA in terms of ATP depletion and its capability to stimulate biliary excretion of residual toxic, endogenous bile acid has been more recently claimed.14 These experiments, however, should be carefully analyzed in terms of the dose of UDCA employed before extrapolation to a clinical situation is attempted. In those studies, mice had been fed 0.5% wt/wt. UDCA, a dose estimated to be equivalent to 30mg/kg per day in humans, based upon the attainment of bile enrichment of UDCA > 75% in these animals.13 However, a similar study in CBDL rats fed one third of that high UDCA dose, equivalent to 10 mg/kg per day in humans (the mean UDCA dose prescribed by us to our patient), afforded protective effects in the CBDL model, as evidenced by reduction of serum ALP, GGT and aminotransferase values.15 Finally, feeding CBDL mice with an even lower UDCA dose, equivalent to 6 mg/kg body weight per day in humans, afforded an intermediate protection, with no change in serum transaminases, but a significant reduction in serum ALP.13 Overall, this illustrates the critical importance of evaluating UDCA dosage in experimental animals before making clinical inferences.
There are several factors that may have limited putative UDCA damaging effects on liver integrity and function in our cholestatic patient. UDCA bioavailability may have been limited by the cholestasis itself.16 UDCA is absorbed by passive non-ionic diffusion in the small intestine, and luminal bile acids facilitate its intestinal absorption.17 Our patient likely had extremely low levels of intestinal bile acids at the start of the UDCA treatment, as suggested by his need for supplementation with vitamin K, a hydrophobic compound that depends on luminal bile acids for intestinal absorption. The improvement in LTs seen when the UDCA dose was increased from 8 to 12 mg/kg/day may reflect, in part, this limited UDCA absorption. Another factor is the adaptive response against bile acid toxicity that the cholestatic patient develops spontaneously.18 This includes downregulation of basolateral bile acid uptake systems and upregulation of basolateral bile acid extrusion pumps, two factors that may have helped to prevent potentially toxic levels of UDCA from being reached in both hepatocytes and the biliary lumen. Supporting this view, the number/size of biliary infarcts induced by high concentrations of UDCA in CBDL mice, which develop an adaptive response against cholestasis similar to humans,18 was extensively reduced when UDCA was administered 3 days after bile duct ligation.14 Our patient received UDCA 3 weeks after the first biochemical indication of cholestasis, a period of time that should have been enough to allow our patient to evoke an adaptive response.
ConclusionIn conclusion, the misleading clinical situation that made us to prescribe UDCA to a patient with biliary obstruction provided us with a unique opportunity to review the widely held contention that UDCA is always detrimental under conditions of biliary obstruction. This assertion is more doubtful in light of the case presented here. In our patient, not only was UDCA not detrimental but also it actually proved beneficial when administered at moderate doses for a short period of time. This should prompt us to reevaluate the association between degree of biliary obstruction and failure of UDCA treatment in chronic obstructive cholangiopathies, and search for alternative explanations of this unsatisfactory therapeutic response. In addition, our case emphasizes the need for re-examination for biliary obstruction in patients with cholestasis when a pattern of increased bilirubin/bile-acid serum levels despite decreased serum liver enzymes arises during UDCA treatment. Finally, our study may help clinicians to better assess the risk-benefit ratio of the use of UDCA in human obstructive cholangiopathies, particularly when relief of the biliary obstruction cannot be achieved by surgical or endoscopic approaches (e.g., multifocal compression of the intrahepatic bile ducts), and when pruritus becomes noticeable.
Abbreviations- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
CBDL: common bile duct-ligated.
- •
CT: computed tomography.
- •
GGT: gamma glutamyl transpeptidase.
- •
PBC: primary biliary cirrhosis.
- •
PSC: primary sclerosing cholangitis.
- •
UDCA: ursodeoxycholic acid.
We thank Dr. Amalia Hidalgo for critical discussion and valuable information on the clinical case.