Background. Incidental hepatocellular carcinoma (iHCC) generates uncertainty over risk of recurrence after liver transplantation (LT). Aim. To compare recurrence between iHCC and confirmed HCC diagnosed prior to transplant based on imaging criteria (cHCC). Material and methods. Fifty-four HCC patients were analyzed from a series of 309 consecutive adult transplanted patients. We developed a recurrence predicting score (RPS) applying ORs based on pathologic risk variables. Results. Incidence of iHCC was 4.8% (n = 15) and overall recurrence 12.9% (cHCC 15.4% and iHCC 7%; P = 0.39). Variables included in the RPS were: microvascular invasion OR 17.8 (1.78-178.97; P = 0.014: 2 points), neural invasion OR 15.5 (1.13-212.17; P = 0.04: 1.5 points), nuclear grade > II OR 9.3 (1.17-74.84; P = 0.035: 1 point), and beyond Up-to 7 criteria OR 13.1 (1.66-103.67; P = 0.015: 1.5 points). Two risk groups were identified: low risk for recurrence (0-1 point) and intermediate-high risk groups (2-6 points). Low risk category remained an independent predictor of recurrence: OR 0.11 (0.01-0.67; P = 0.017); AUROC of 0.75 (0.54-0.96). A tendency towards more patients categorized as low risk group among iHCC patients was observed (69.2%; P = 0.13). Conclusions. In this series iHCC was not associated to lower risk of recurrence when compared to cHCC. We propose application of an RPS as a clinical tool for recurrence risk estimation.
Recent data from liver transplant centers in Europe and the United States have shown that approximately 30% of all liver transplants (LT) are indicated for hepatocellular carcinoma (HCC).1 Currently, with routine HCC monitoring (ultrasound exam every 6 months), approximately 30% of patients are diagnosed at early stages of disease, making them candidates for curative treatments.2The Milan criteria have been accepted for selecting HCC LT candidates.3,4 However, despite their application, recurrence after transplantation is diagnosed in 15-40% of the cases.5–7 Once established, recurrence is considered to be systemic with poor prognosis and limited cure.5 Known risk factors for recurrent HCC include: number of tumor lesions, total tumor diameter (TTD), pre-transplant serum alpha-fetoprotein (AFP) level, tumor progression while on the waiting list,6 presence of microvascular invasion and tumor differentiation grade found in explanted liver specimen.7
Advances in diagnostic imaging have enhanced HCC detection rates. Most tumor lesions are diagnosed before LT. However, very small nodules may only be detected during explant pathology examination. When Incidental HCC (iHCC) is thus detected, uncertainty regarding risk of recurrence and patient follow-up management after transplant ensues. While iHCC incidence varies in different published series between 2 and 40%, with a mean of 16%,8–10 controversy exists regarding the risk of recurrence and overall patient survival among iHCC patients and those with confirmed HCC diagnosed prior to transplant based on imaging criteria (cHCC). The aim of this study was to evaluate iHCC incidence during the Milan era, identify risk factors for recurrence and compare recurrence rates between iHCC and cHCC in a cohort of transplanted patients. We hypothesized that incidental tumors would present lower rates of microvascular invasion and smaller undifferentiated nodules and therefore less recurrence.
Material and MethodsA total of 309 adult (> 17 years of age) liver transplants were consecutively performed at the Hospital Universitario Austral and the Hospital Italiano of Buenos Aires between June 1st 2005 and June 30th 2010. Patients who did not present cirrhosis or were transplanted for fulminant hepatic failure were excluded from this analysis. At both centers and as established by international guidelines,14 pre-LT monitoring for HCC was performed on all patients using ultrasound with or without serum AFP assay every 6 months. HCC imaging diagnosis was established based on the presence of arterial enhancement and washout during late portal phase on computed tomography (CT) or magnetic resonance imaging (MRI). In cases not presenting typical vascular pattern on CT or MRI scans, fine needle biopsy was performed to confirm or exclude HCC.14 Both number and corresponding diameter of nodules were recorded in all cHCC cases, which were then assigned scores according to Milan3 and University of California San Francisco criteria (UCSF).15–17
Medical records were reviewed for clinical and recipient characteristics as well as most recent dynamic tumor images and laboratory data prior to transplant. Immediate pre-transplant Child Pugh and Model for End-stage Liver Disease (MELD) scores were calculated.
Follow-up visit scheduling varied between centers but CT or MRI, bone scans and serum AFP assay were performed every 6 months in all patients. Tumor recurrence was determined based on imaging, serum AFP or biopsy when available. Metastasis location and size as well as serum AFP at time of recurrence were recorded. Recurrence-free survival was estimated as time elapsed between LT and date of recurrence.
Immunosuppression was performed according to independent selection criteria at each hospital. Initial immunosuppression in both centers included methylprednisolone 1,200 mg during the first 24 h after transplant, followed by tacrolimus (Tac) or cysclosporine A (CsA) with or without micophenolate sodium/mophetil (MMF).
Pathology findingsTwo different pathologists examined the explanted liver specimens at each site. Macroscopy results (10 mm slides) were reported by the same pathologist in each center. Microscopic evaluation for each nodule was conducted to characterize tumor biology including:
- •
Background fibrosis and inflammation. Confirmation of cirrhosis.
- •
Number and diameters (cm) of HCC nodules.
- •
Largest tumor size, “major nodule”.
- •
Presence of microvascular invasion: defined as tumor cell invasion of either vascular (artery or vein) or lymphatic vessels on standard H&E stained slides.
- •
Presence of neural invasion: defined as tumor cell invasion of neural structures or nerves on standard H&E stained slides.
- •
Nuclear grade: assessed using the modified Edmonson Steiner system.24
If a patient had previously known HCC and more nodules were found in the explanted liver analysis, these nodules were not categorized as “incidental HCC”.
Statistical analysisComparison between iHCC and cHCC characteristics was performed using Fisher’s test for dichotomous variables (expressed as frequencies) and Mann Whitney-U test for continuous variables (medians and interquartile range). Univariate analysis, using logistic regression was applied in order to identify significant variables related to recurrent HCC. All HCC transplanted patients with at least 6 months follow up were included for the logistic regression analysis. Those pathologic variables related with recurrent HCC on univariate analysis were included in order to develop a score to determine probability of recurrence after transplant. All univariate variables with P values < 0.1 were considered for multivariate analysis; models were generated by stepwise backward elimination, using P values < 0.05 to remove variables not significantly associated with the outcome. To assess the model’s goodness of fit and discriminatory power for patients presenting or not HCC recurrence, an area under the receiver operating characteristic curve (AUROC) was generated. Finally, Kaplan Meier analysis for overall survival and recurrence free survival in cHCC and iHCC subgroups was calculated. Data collected was stored in a database and analyzed by Stat View for Windows, Abacus Concepts (STATA version 10.1).
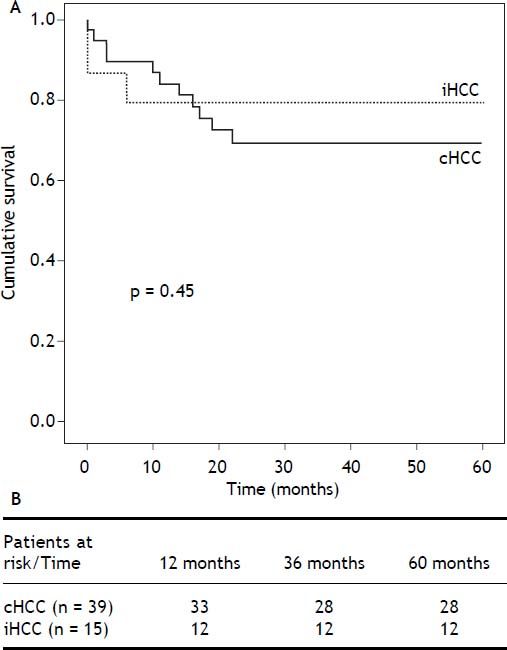
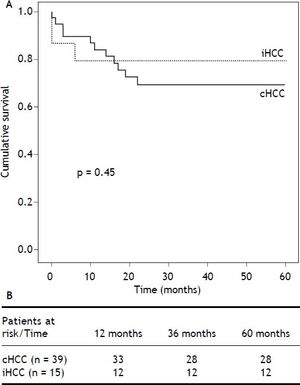
ResultsA total of 309 adult cirrhotic patients were consecutively transplanted during the study period. All patients had an elective LT and underwent ultrasound HCC screening every six months in both centers. Of these, 54 patients had HCC (17.5%), 39 (72%) cHCC and 15 (28%) iHCC. Four patients had false positive cHCC diagnosis (n = 2 regenerative nodules, n = 1 biliary hamarthoma and n = 1 cholangiocarcinoma) and were excluded from the final analysis. Cumulative iHCC incidence was 4.8% (n = 15/309). Overall median patient survival was 68% after a mean follow-up of 4.5 years; no differences in patient survival were found between iHCC and cHCC (P = 0.45) (Figure 1).
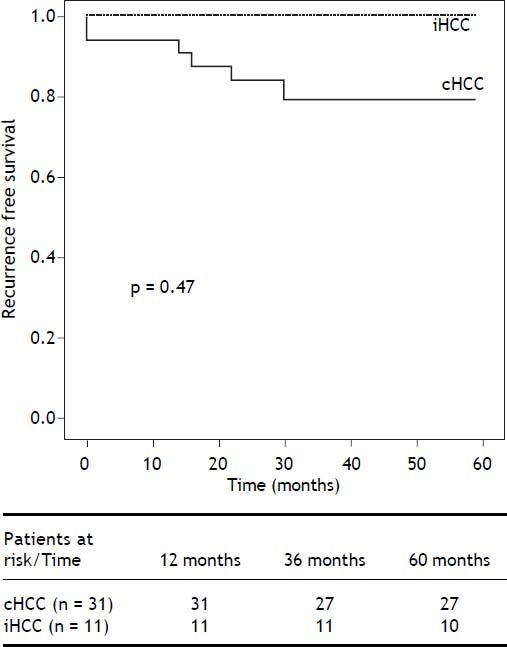
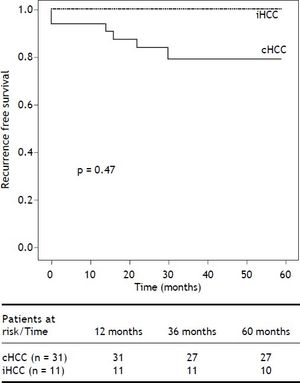
Overall risk for recurrence was assessed after excluding 10 patients who died within 6 months of LT. Of the 44 remaining patients (iHCC n = 11, cHCC n = 33), recurrent HCC was diagnosed in 7, with a cumulative incidence of 15.9% and a mean follow up of 4.5 years (3.7-5.2 years, 95% CI). Overall recurrence free survival was 84.1% (52.6-93.6%). Recurrence was diagnosed in 6 patients within 2 years of LT, while the remaining patient recurred after 3.6 years of follow-up (Figure 2). Among patients presenting recurrence, progressive and endstage cancer disease was the cause of death in 5, 1 patient died of sepsis and one patient with late HCC recurrence is still alive. At the time of HCC recurrence, metastases were diagnosed in 4 patients (57%). Of the 7 recurring patients, 4 (57%) developed both hepatic and extrahepatic tumors with higher serum AFP levels (mean 5,368 ng/mL) and 3 (42%) developed isolated extrahepatic lesions with lower serum AFP levels (mean 1,127 ng/mL). The most frequent metastatic location was bone in 71% (n = 10) of the cases.
Kaplan Meier recurrence free survival analysis. Overall risk for recurrence was assessed after excluding 10 patients who died within 6 months of LT. Of the 44 remaining patients (iHCC n = 11, HCC n = 33), recurrent HCC was diagnosed in 7, with a cumulative incidence of 15.9%, after a mean follow up of 4.5 years. Although not statistically significant, cumulative recurrence was lower in iHCC patients (7% and 15.4%, P = 0.47). Overall recurrence free survival was 84.1% (52.6-93.6%,).
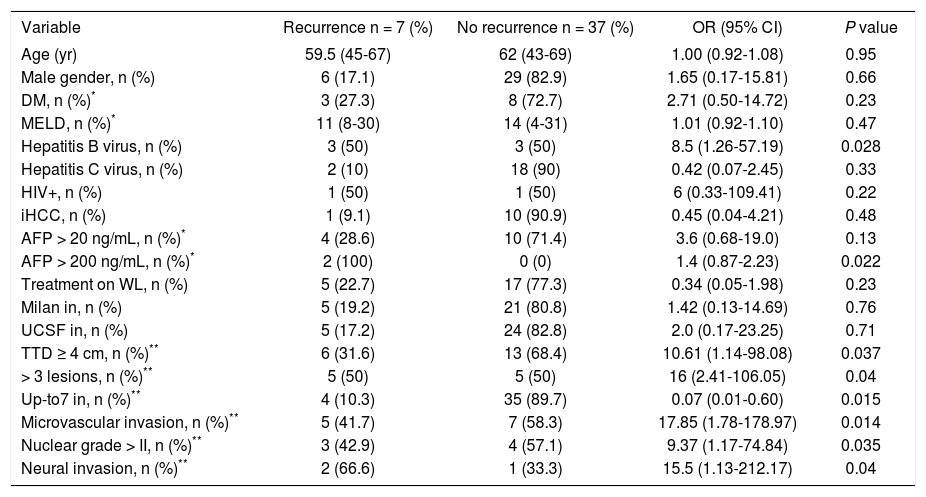
Explanted liver pathology findings related to HCC recurrence included: TTD > 4 cm OR 10.6 (1.14-98.08; P = 0.037); > 3 HCC lesions OR 16 (2.41-106.05; P = 0.04); presence of microvascular invasion OR 17.8 (1.78-178.97; P = 0.014); nuclear grade > II OR 9.3 (1.17-74.84; P = 0.035); presence of neural invasion OR 15.5 (1.13-212.17; P = 0.04) and not meeting Up-to 7 criteria OR 13.1 (1.66-103.67; P = 0.015).
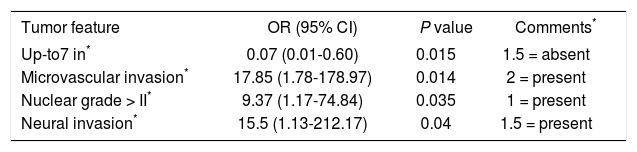
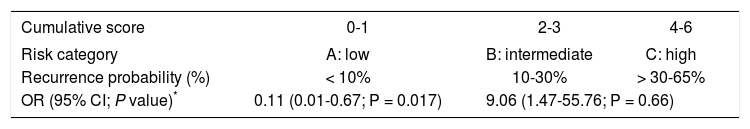
HCC recurrence predicting scoreRisk variables for recurrence are shown in table 3. Based on a univariate regression analysis we developed a recurrence predicting score (RPS) using only pathology risk factors for recurrence. Points were assigned to each variable and divided by 9 (lowest OR of factors to simplify score calculation). Scores of 2, 1.5 and 1 point corresponded to presence of microvascular invasion, neural invasion and nuclear grade > II, respectively. Not meeting Up-to 7 criteria was assigned 1.5 points. This generated a RPS ranging from 0 to 6 points and identified two distinct groups: group A or low risk (0-1 point) with 6.5% cumulative risk for recurrent HCC (n = 2/31) and group B or intermediate-high (n = 5/13) risk group (2-6 points) with 14.3 and 66.7% cumulative risk of recurrence, respectively. Only the low risk category remained an independent predictor of recurrence after a multivariate logistic regression analysis with OR 0.11 (0.01-0.67; P = 0.017) and ROC curve analysis predicting non-recurrence at 0.75 (0.54-0.96) (Tables 4-5).
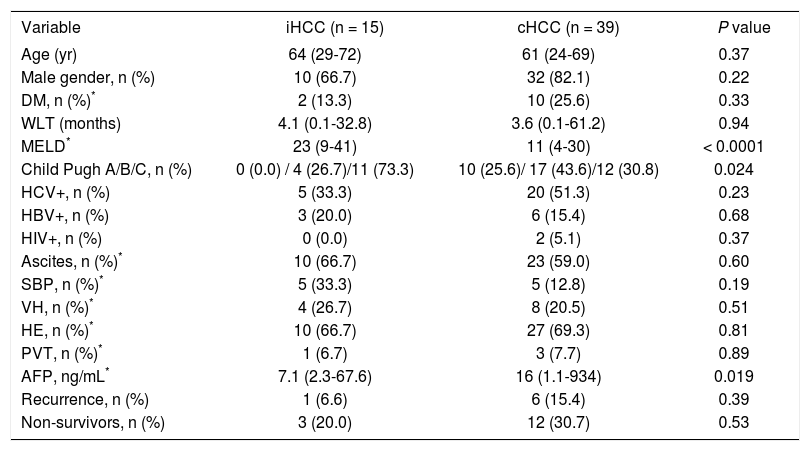
Comparative analysis between incidentally found hepatocellular carcinoma and hepatocellular carcinoma diagnosed prior to transplantNo significant differences were observed regarding patients age between iHCC and cHCC. iHCC patients had higher Child Pugh (P = 0.024) and Meld scores at the time of transplant (24 vs. 12.4, P < 0.0001). On the other hand, mean serum AFP levels were higher among patients with cHCC (87.6 vs. 12.8 ng/mL, P = 0.019); only 2 patients (13.3%) with iHCC had serum AFP > 20 ng/mL (P = 0.075) (Table 1).
Patients’ baseline and clinical characteristics.
| Variable | iHCC (n = 15) | cHCC (n = 39) | P value |
|---|---|---|---|
| Age (yr) | 64 (29-72) | 61 (24-69) | 0.37 |
| Male gender, n (%) | 10 (66.7) | 32 (82.1) | 0.22 |
| DM, n (%)* | 2 (13.3) | 10 (25.6) | 0.33 |
| WLT (months) | 4.1 (0.1-32.8) | 3.6 (0.1-61.2) | 0.94 |
| MELD* | 23 (9-41) | 11 (4-30) | < 0.0001 |
| Child Pugh A/B/C, n (%) | 0 (0.0) / 4 (26.7)/11 (73.3) | 10 (25.6)/ 17 (43.6)/12 (30.8) | 0.024 |
| HCV+, n (%) | 5 (33.3) | 20 (51.3) | 0.23 |
| HBV+, n (%) | 3 (20.0) | 6 (15.4) | 0.68 |
| HIV+, n (%) | 0 (0.0) | 2 (5.1) | 0.37 |
| Ascites, n (%)* | 10 (66.7) | 23 (59.0) | 0.60 |
| SBP, n (%)* | 5 (33.3) | 5 (12.8) | 0.19 |
| VH, n (%)* | 4 (26.7) | 8 (20.5) | 0.51 |
| HE, n (%)* | 10 (66.7) | 27 (69.3) | 0.81 |
| PVT, n (%)* | 1 (6.7) | 3 (7.7) | 0.89 |
| AFP, ng/mL* | 7.1 (2.3-67.6) | 16 (1.1-934) | 0.019 |
| Recurrence, n (%) | 1 (6.6) | 6 (15.4) | 0.39 |
| Non-survivors, n (%) | 3 (20.0) | 12 (30.7) | 0.53 |
Normal values: Alpha-fetoprotein 0.6-4.4 ng/mL.
Pre-LT variables. Dichotomous variables are shown as percentage and continuous variables are shown as median (interquartile range). AFP: alpha-fetoprotein. DM: diabetes mellitus. HBV: hepatitis B virus. HCV: hepatitis C virus. HE: hepatic encephalopathy. HIV: human immunodeficiency virus. MELD: Model for End-stage Liver Disease. PVT: pre-LT portal vein thrombosis. SBP: spontaneous bacterial peritonitis. VH: variceal hemorrhage. WLT: waiting list time.
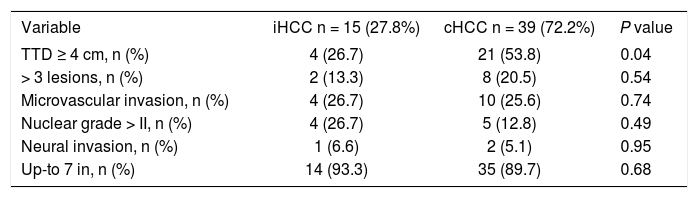
Although not statistically significant, cumulative recurrence was lower in iHCC patients (7 and 15.4%, P = 0.47). Among explanted liver analysis, we observed incidental tumors were smaller (TTD ≥ 4 cm 26.7 vs. 53.8%; P = 0.049) with fewer nodules (> 3 lesions 13.3% vs. 20.5%; P = 0.54) and 93.3% were within Up-to 7 criteria when compared to cHCC. However, iHCC and cHCC tumors had similar levels of microvascular (26.7 vs. 25.6%; P = 0.74) and neural invasion (6.6 vs. 5.1%; P = 0.95) (Table 2). RPS identified 9/13 iHCC (69.2%) and 20/31 cHCC patients (64.5%) as low risk for R (P = 0.13).
Explanted liver pathologic analysis.
| Variable | iHCC n = 15 (27.8%) | cHCC n = 39 (72.2%) | P value |
|---|---|---|---|
| TTD ≥ 4 cm, n (%) | 4 (26.7) | 21 (53.8) | 0.04 |
| > 3 lesions, n (%) | 2 (13.3) | 8 (20.5) | 0.54 |
| Microvascular invasion, n (%) | 4 (26.7) | 10 (25.6) | 0.74 |
| Nuclear grade > II, n (%) | 4 (26.7) | 5 (12.8) | 0.49 |
| Neural invasion, n (%) | 1 (6.6) | 2 (5.1) | 0.95 |
| Up-to 7 in, n (%) | 14 (93.3) | 35 (89.7) | 0.68 |
Only explanted liver variables. Dichotomous variables are shown as percentage. TTD: total tumor diameter (sum of diameters of all HCC nodules). Up-to7: sum of number of all HCC nodules + diameter of biggest HCC nodule.
Risk variables for hepatocellular carcinoma recurrence excluding deceased patients at 6 months of follow up (n = 10).
| Variable | Recurrence n = 7 (%) | No recurrence n = 37 (%) | OR (95% CI) | P value |
|---|---|---|---|---|
| Age (yr) | 59.5 (45-67) | 62 (43-69) | 1.00 (0.92-1.08) | 0.95 |
| Male gender, n (%) | 6 (17.1) | 29 (82.9) | 1.65 (0.17-15.81) | 0.66 |
| DM, n (%)* | 3 (27.3) | 8 (72.7) | 2.71 (0.50-14.72) | 0.23 |
| MELD, n (%)* | 11 (8-30) | 14 (4-31) | 1.01 (0.92-1.10) | 0.47 |
| Hepatitis B virus, n (%) | 3 (50) | 3 (50) | 8.5 (1.26-57.19) | 0.028 |
| Hepatitis C virus, n (%) | 2 (10) | 18 (90) | 0.42 (0.07-2.45) | 0.33 |
| HIV+, n (%) | 1 (50) | 1 (50) | 6 (0.33-109.41) | 0.22 |
| iHCC, n (%) | 1 (9.1) | 10 (90.9) | 0.45 (0.04-4.21) | 0.48 |
| AFP > 20 ng/mL, n (%)* | 4 (28.6) | 10 (71.4) | 3.6 (0.68-19.0) | 0.13 |
| AFP > 200 ng/mL, n (%)* | 2 (100) | 0 (0) | 1.4 (0.87-2.23) | 0.022 |
| Treatment on WL, n (%) | 5 (22.7) | 17 (77.3) | 0.34 (0.05-1.98) | 0.23 |
| Milan in, n (%) | 5 (19.2) | 21 (80.8) | 1.42 (0.13-14.69) | 0.76 |
| UCSF in, n (%) | 5 (17.2) | 24 (82.8) | 2.0 (0.17-23.25) | 0.71 |
| TTD ≥ 4 cm, n (%)** | 6 (31.6) | 13 (68.4) | 10.61 (1.14-98.08) | 0.037 |
| > 3 lesions, n (%)** | 5 (50) | 5 (50) | 16 (2.41-106.05) | 0.04 |
| Up-to7 in, n (%)** | 4 (10.3) | 35 (89.7) | 0.07 (0.01-0.60) | 0.015 |
| Microvascular invasion, n (%)** | 5 (41.7) | 7 (58.3) | 17.85 (1.78-178.97) | 0.014 |
| Nuclear grade > II, n (%)** | 3 (42.9) | 4 (57.1) | 9.37 (1.17-74.84) | 0.035 |
| Neural invasion, n (%)** | 2 (66.6) | 1 (33.3) | 15.5 (1.13-212.17) | 0.04 |
Normal values: alpha-fetoprotein 0.6-4.4 ng/mL.
Explanted liver variables. Dichotomous variables are shown as percentage and continuous variables are shown as median (range). AFP: alpha-fetoprotein. DM: diabetes mellitus. HE: hepatic encephalopathy. HIV: human immunodeficiency virus. iHCC: incidentally found hepatocellular carcinoma. MELD: Model for End-stage Liver Disease. OR: odds ratio. TTD: total tumor diameter (sum of diameters of all HCC nodules). Up-to7: sum number of all HCC nodules + diameter of biggest HCC nodule. UCSF: University of California, San Francisco. WL: waiting list.
Predicting recurrence score (PRS): pathological assessment.
Cumulative score-recurrence probability.
| Cumulative score | 0-1 | 2-3 | 4-6 |
|---|---|---|---|
| Risk category | A: low | B: intermediate | C: high |
| Recurrence probability (%) | < 10% | 10-30% | > 30-65% |
| OR (95% CI; P value)* | 0.11 (0.01-0.67; P = 0.017) | 9.06 (1.47-55.76; P = 0.66) | |
Incidental HCC remains an unresolved problem. Before the study, we believed these tumors might have presented lower recurrence rate as a result of less aggressive tumor biology. However, this was not the case as iHCC presented almost the same rate of microvascular invasion and higher nuclear Edmonson grade than cHCC. Our data supports the notion that HCC monitoring should remain strict,2 and surveillance failure will uncover incidental tumors after LT.
Incidence of iHCC in our cohort was 4.8%, lower than that observed in previously reported series;8–10 indicating a correct implementation of HCC surveillance in the pre-transplant setting in both LT units, as all of the patients were screened using ultrasound every 6 months as recommended in clinical practice guidelines.14 Castillo, et al. reported only 9 and 38% ultrasound screening for cHCC and iHCC patients, respectively.10 Sotiropoulus and co-workers proposed a new definition of iHCC. These authors recommend using more detailed radiological evaluation (CT or MRI) and stricter time windows for imaging prior to LT to increase HCC detection,11 even though using CT or MRI scans is not recommended for routine patient monitoring.2
Since implementation of the MELD score began in Argentina (July 2005), an additional 22 points are allotted to HCC falling within Milan criteria. Data from the Argentine National Registry for Organ Donation and Ablation (INCUCAI) indicate the median MELD score for LT patients in our country is 24. HCC patients with 22 extra points undergo LT between 2 and 4 months after obtaining these additional points.25 We also observed that patients with iHCC had more advanced liver disease than cHCC patients at time of transplantation.10
Pathologic analysis of the explanted liver remains a key component for recurrence risk assessment. Tumor differentiation or nuclear grade evaluated by Edmonson-Steiner grading,24 presence of microvascular and perineural invasion, size and number of nodules are all classic risk factors.18,20 Although presence of microvascular invasion is an accurate predictor of recurrence, it does not ensure recurrence in itself. Furthermore, a combination or sum of risk factors seems to be necessary for recurrence to develop.19 In this sense, Mazzaferro, et al. have proposed a model defined as Up-to 7 criteria in relation to number of tumors and major nodule diameter.21,22 Risk variables analyzed in our cohort included the Up-to 7 criteria, microvascular invasion, nuclear grade, total tumor diameter and number of nodules, which we then used to establish RPS, and thus develop an accessible clinical tool for predicting recurrence.
Given the retrospective design of the study, we were not able to include additional explanted liver variables in our study, such as immunohistochemical biomarkers or different cancer pathways, microsatellitosis or presence of giant or bizarre cells in > 25% of the tumor.20 However, these signs are not widely accepted as risk factors for HCC recurrence as Parfitt, et al., proposed.20 Firstly, there is no clear consensus on the definition of microsatellitosis, defined as microscopic multifocality or a satellite nodule. Additionally, to the best of our knowledge, presence of giant or bizarre cells > 25% of the tumor is not a clear risk factor for recurrence and probably has a wide range of interobserver agreement.20 Finally, those giant or bizarre cells are categorized as markedly anaplastic nuclei among the Edmonson Steiner grading system.24 Other authors proposed a different predicting recurrence score, which includes explanted liver variables such as microvascular invasion, major nodule diameter, bilobar presentation and nuclear grade.19 Most of the explanted liver analysis in our study included conventional variables such as number and diameter of lesions, microvascular invasion, nuclear grade and Up-to-7 criteria. The score proposed by Parfitt et al has been published earlier than that of Mazzaferro, et al., (Up-to-7 criteria) and it has been assessed from only 1 liver transplant program among 75 patients. The Up-to-7 criteria have been assessed from a retrospective multicenter explanted liver analysis of more than a thousand patients and it was published in the year 2009.21 In this sense, we tried to analyze the Up-to-7 criteria in our cohort rather than additional and non-conventional risk factors because we considered that Mazzaferro’s score has been widely accepted.22
Traditionally, lower incidence of tumor recurrence and improved survival has been described in iHCC patients, but conflicting reports have also been published.8 A low risk of recurrence may be attributable, at least in part, to more favorable tumor biology. Reported prevalence of microvascular invasion for iHCC is 15%, which depending on the series is both lower10,23 and higher7,13 than rates published for cHCC. Unexpectedly, iHCC patients in this cohort had higher nuclear grade tumors (26.7 vs. 12.8%) and a similar rate of microvascular invasion to cHCC (26.7 and 25.6%). This observation raises concern about the real risk of recurrence among incidental tumors. However, after applying PRS, we did not observe differences in numbers of patients with low or intermediate-high risk of recurrence between iHCC and cHCC.
Finally, the iHCC group showed a tendency towards better survival after transplant, which cHCC patients in our cohort did not. Castillo, et al. showed lower survival in the iHCC group,10 Charco, et al. showed no differences,13 while Sotiropoulus, et al. found discordant results in their meta-analysis.11
Our findings do have some limitations. Firstly, the comparison between iHCC and cHCC is based on a retrospective analysis including a small number of patients. Secondly, the proposed scoring system, albeit clinically relevant, needs to be further validated in a larger prospective cohort of HCC patients. Furthermore, it would be of interest to analyze molecular cancer pathways as risk factors of recurrence in these patients.
In summary, our data suggests that recurrence may be lower in some patients with iHCC compared to cHCC patients. We therefore propose applying a scoring system, the RPS, to better assess this risk. Prospective surveillance studies including iHCC are needed to validate the RPS and to propose a cost-effective follow-up for HCC recurrence after LT.
Abbreviations- •
AFP: alpha-fetoprotein (AFP).
- •
cHCC: confirmed HCC diagnosed prior to transplant (imaging criteria).
- •
CI: confidence interval.
- •
CNI: calcinurin inhibitors.
- •
CsA: cysclosporine A.
- •
CT: computerized tomography.
- •
HCC: hepatocellularcarcinoma.
- •
iHCC: incidentally found hepatocellular carcinoma.
- •
LT: liver transplantation.
- •
MELD: Model for End-stage Liver Disease.
- •
MMF: mophetilmicophenolate.
- •
MRI: magnetic resonance imaging.
- •
mTOR: mammalian target inhibitors.
- •
OR: Odds Ratio.
- •
PRS: predicting recurrence score.
- •
PVT: portal vein thrombosis.
- •
Tac: tacrolimus.
- •
TTD: total tumor diameter.
The manuscript is presented in memory of Dr. Carlos Rowe (R.I.P), esteemed colleague and friend. We thank Kathleen Dowd and C Podestá for their assistance with editing this paper.
Authors Contribution- •
Federico Piñero. Study concept-design, acquisition of data, analysis and interpretation of data, drafting of the manuscript.
- •
Manuel Mendizabal. Critical revision of the manuscript and statistical analysis.
- •
Paola Casciato. Critical revision of the manuscript.
- •
Omar Galdame. Critical revision of the manuscript.
- •
Rodolfo Quiros. Analysis and interpretation of data and statistical analysis.
- •
Juan Bandi. Critical revision of the manuscript.
- •
Eduardo Mullen. Critical revision of the manuscript.
- •
Oscar Andriani. Critical revision of the manuscript.
- •
Eduardo de Santibañes. Critical revision of the manuscript.
- •
Luis G Podestá. Critical revision of the manuscript.
- •
Adrian Gadano. Critical revision of the manuscript.
- •
Marcelo Silva. Critical revision of the manuscript.
This research received no specific grant from any funding agency in the public, commercial, or not-forprofit sectors. All authors stated no conflict of interest regarding this manuscript.