Background. Liver cirrhosis is associated with intestinal epithelial barrier dysfunction, which may be affected by oxidative stress. Studies in cirrhotic rats provided evidence for intestinal oxidative stress, but studies in cirrhotic patients are scarce. We have shown intestinal barrier dysfunction in patients with compensated cirrhosis.
Aim. The present study aimed to investigate whether oxidative stress occurs in the intestinal mucosa of compensated cirrhotic patients and may contribute to barrier dysfunction.
Material and methods. Oxidative stress was studied in duodenal and sigmoid biopsies from 15 cirrhotic patients and 22 controls by analyzing transcription of genes involved in glutathione and uric acid metabolism using quantitative real-time polymerase chain reaction. Protein levels of glutathione and glutathione disulphide were measured and the glutathione/glutathione disulphide ratio was calculated as marker of oxidative stress. In addition, intestinal myeloperoxidase and fecal calprotectin were determined.
Results. Gene transcription of glutathione synthetase and glutathione reductase were significantly different in duodenal and sigmoid biopsies of cirrhotic patients vs. controls, but no alterations were found for other genes nor for glutathione, glutathione disulphide, glutathione/glutathione disulphide ratio and intestinal myeloperoxidase and fecal calprotectin concentrations.
Conclusion. This study did not find indications for oxidative stress and low-grade inflammation in the small and large intestine of stable compensated cirrhotic patients. Although these preliminary findings need further validation, we found intestinal oxidative stress not to be a major mechanism contributing to epithelial barrier dysfunction in patients with compensated cirrhosis.
Cirrhosis is the end stage of chronic liver diseases and is associated with a high morbidity and mortality. Increasing evidence indicates that the gastrointestinal (GI) tract plays a role in the development of liver cirrhosis and its complications. Cirrhosis has been associated with both structural and functional alterations in the GI tract, such as edema of lamina propria,1 distended intercellular spaces,2 impaired motility,3 changes in microbiota composition4 and dysfunction of the epithelial barrier. Several studies have shown an increased intestinal permeability in patients with liver cirrhosis.5–7 This may facilitate translocation of bacteria and bacterial products such as endotoxin into the systemic circulation,8 and thereby contribute to the development of cirrhotic complications, such as spontaneous bacterial peritonitis.9 Epithelial barrier function is regulated by intercellular tight junctions (TJ) and adherens junctions (AJ). Recently, Assimakopoulos, et al.10 and Du Plessis, et al.,11 found alterations in the expression of TJ proteins in duodenal biopsies of compensated and decompensated cirrhotic patients.
Oxidative stress is a potential mechanism underlying intestinal epithelial barrier dysfunction as it can induce direct epithelial cell damage and disrupt TJ and/or AJ function and structure.12–15 Possible factors causing oxidative damage in the intestine of patients with cirrhosis include ingested alcohol,16 changes in intestinal microbiota,4,17,18 intestinal inflammation11,19 and disturbed microcirculation of the intestinal mucosa secondary to portal hypertension.1,20,21 In addition, systemic inflammation and oxidative stress as well as oxidative stress inducers produced in the liver, may be transferred to the intestine via blood and bile.15,22 These factors together with a decreased antioxidant status in cirrhotic patients23 may promote intestinal oxidative damage.
Few studies in rats with carbon tetrachloride (CCl4)-induced cirrhosis have provided evidence for oxidative stress in both the small and large intestine, by e.g. increased xanthine oxidase (XO) activity, increased malondialdehyde (MDA) levels, and alterations in antioxidant status, such as low levels of reduced glutathione (GSH) and high levels of glutathione disulphide (GSSG).13,24–26 So far, one recently published study has assessed mucosal proliferation, apoptosis and oxidative stress in duodenal biopsies of patients with cirrhosis and found a significantly increased intestinal lipid peroxidation (i.e. lipid hydroperoxides) as well as increased plasma endotoxin concentrations.27
In a previous study we have investigated the intestinal epithelial barrier function in patients with compensated liver cirrhosis in order to find out whether an increased intestinal permeability may be a risk factor for the progression towards decompensated cirrhosis and observed an increased small intestinal permeability in a subgroup of patients with alcohol-related cirrhosis and an increased large intestinal permeability in the whole group of compensated cirrhotic patients.28
We hypothesized that intestinal oxidative stress is involved in the epithelial barrier dysfunction of patients with compensated liver cirrhosis. Aim of the present study was therefore to investigate the occurrence of oxidative stress not only in the mucosa of the small, but also of the large intestine in patients with compensated cirrhosis and to compare data with those obtained in healthy controls.
Material and MethodsPatients and study designA subgroup of compensated cirrhotic patients (i.e. without clinically evident complications, including ascites, variceal hemorrhage, hepatic encephalopathy and/ or jaundice) and a control group of healthy volunteers participating in a prior prospective case-control study on intestinal permeability were available for analyses of intestinal oxidative stress. The design and clinical details of the study have been described elsewhere.28 Briefly, 15 cirrhotic patients and 22 controls underwent a gastroduodenoscopy and/or sigmoidoscopy after an overnight fast without prior bowel cleansing. Mucosal biopsies were obtained from standardized locations: the second segment of the duodenum and the sigmoid approximately 20 cm from the anal sphincter, respectively. Biopsies for gene transcription and protein expression were snap frozen in liquid nitrogen and stored at −80 °C until further analyses. Biopsies for histological evaluation of haema-toxylin and eosin (H&E) stained sections by one experienced pathologist, were fixed in 4% formaldehyde and embedded in paraffin.
In addition, subjects collected a fresh fecal sample in a sterile container. Aliquots were stored within 12 h after defecation at −80 °C until further analysis.
The study has been approved by the Medical Ethics Committee of Maastricht University Medical Center (MUMC), conducted according to the revised version of the Declaration of Helsinki (October 2008, Seoul) and registered at the US National Library of Medicine (http://www.clinicaltrials.gov, NTC01081236). All subjects (patients and healthy volunteers) gave their written informed consent before participation.
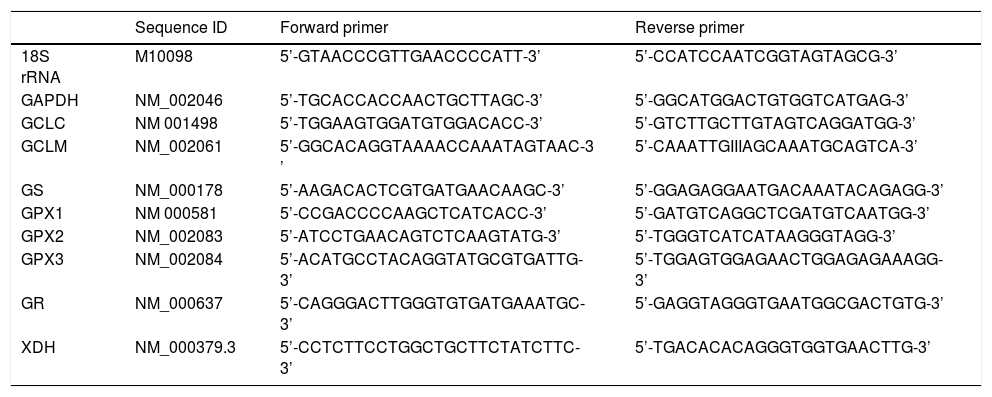
Transcription of oxidative stress-related genesTranscription of genes involved in glutathione (GSH) and uric acid metabolism, i.e. glutamate-cysteine ligase, catalytic subunit (GCLC), glutamate-cysteine ligase, modifier subunit (GCLM), glutathione synthetase (GS), glutathione peroxidases (GPX1, GPX2 and GPX3), glutathione reductase (GR), and xanthine dehydrogenase (XDH), in mucosal biopsies were determined by qRT-PCR. Total RNA was isolated from the frozen biopsies using TRIzol reagent (Invitrogen, Carlsbad, USA) and purified with the RNeasy Mini Kit (Qiagen, Venlo, the Netherlands). The concentration of purified RNA was measured with the NanoDrop. Finally, 500 ng total RNA was used as a template for the cDNA reaction, which was synthesized using the iScriptcDNA Synthesis Kit (Bio-Rad, Veenendaal, the Netherlands). The cDNA was diluted to a concentration of 4 ng/μL. Each reaction contained 5 μL cDNA template solution, 12.5 μL iQ SYBR Green Supermix (Bio-Rad), 1 μL forward and reverse primers (10 μM) and 5.5 μL sterile water. Primer sequences have been listed in table 1. Housekeeping genes included were 18S rRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Reactions were run on the My IQ Single Color RT-PCR Detection System (Bio-Rad). PCR conditions used were 3 min at 95 °C, followed by 40 amplification cycles of 10 sec at 95 °C and 45 sec at 60 °C. Data were expressed as normalized expression ratios.
Primer sequences of housekeeping genes and genes involved in glutathione and uric acid metabolism.
| Sequence ID | Forward primer | Reverse primer | |
|---|---|---|---|
| 18S rRNA | M10098 | 5’-GTAACCCGTTGAACCCCATT-3’ | 5’-CCATCCAATCGGTAGTAGCG-3’ |
| GAPDH | NM_002046 | 5’-TGCACCACCAACTGCTTAGC-3’ | 5’-GGCATGGACTGTGGTCATGAG-3’ |
| GCLC | NM 001498 | 5’-TGGAAGTGGATGTGGACACC-3’ | 5’-GTCTTGCTTGTAGTCAGGATGG-3’ |
| GCLM | NM_002061 | 5’-GGCACAGGTAAAACCAAATAGTAAC-3 ’ | 5’-CAAATTGIIIAGCAAATGCAGTCA-3’ |
| GS | NM_000178 | 5’-AAGACACTCGTGATGAACAAGC-3’ | 5’-GGAGAGGAATGACAAATACAGAGG-3’ |
| GPX1 | NM 000581 | 5’-CCGACCCCAAGCTCATCACC-3’ | 5’-GATGTCAGGCTCGATGTCAATGG-3’ |
| GPX2 | NM_002083 | 5’-ATCCTGAACAGTCTCAAGTATG-3’ | 5’-TGGGTCATCATAAGGGTAGG-3’ |
| GPX3 | NM_002084 | 5’-ACATGCCTACAGGTATGCGTGATTG-3’ | 5’-TGGAGTGGAGAACTGGAGAGAAAGG-3’ |
| GR | NM_000637 | 5’-CAGGGACTTGGGTGTGATGAAATGC-3’ | 5’-GAGGTAGGGTGAATGGCGACTGTG-3’ |
| XDH | NM_000379.3 | 5’-CCTCTTCCTGGCTGCTTCTATCTTC-3’ | 5’-TGACACACAGGGTGGTGAACTTG-3’ |
18S rRNA: 18S ribosomal RNA. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. GCLC: glutamate-cysteine ligase, catalytic subunit GCLM: glutamatecysteine ligase, modifier subunit GS: glutathione synthetase. GPX: glutathione peroxidase. GR: glutathione reductase. XDH: xanthine dehydrogenase.
Frozen biopsies for oxidative stress analyses were ground with a mortar and pastel cooled in liquid nitrogen, and resuspended in 220 μL ice-cold milliQ. Two hundred μL from this suspension was added to 20 μL of an acidic buffer (13% 5-Sulfosalicilic acid, 100 mmol/LHCl in PBS). After centrifugation, the supernatant was used to determine the concentrations of GSH and GSSG using the method described by Julicher, et al.29 Frozen biopsies for myeloperoxidase (MPO) analyses were ground with a mortar and pastel cooled in liquid nitrogen, and resuspended in 100 μL ice-cold PBS containing 10 μL/mL Protease Inhibitor Cocktail (Sigma-Aldrich, Zwijndrecht, the Netherlands). After centrifugation for 20 min (10.000 rpm, 4 °C), the supernatant was stored at −80 °C until further analysis. MPO in supernatant was determined using an ELISA Kit (HBT, Uden, the Netherlands) according to the manufacturer’s instructions. Total protein content in the supernatants was quantified using the BCA Protein Assay Kit (PierceTM, Rockford IL, USA).
GSH and GSSG were expressed as nmol/mg protein in biopsies and the GSH/GSSG ratio was calculated as a marker of oxidative stress. MPO was expressed as ;μg/g protein in biopsies.
Fecal calprotectinApproximately 100 mg of wet weight feces was diluted 50 times in extraction buffer (0.1 mol/L Tris, 0.15 mol/L NaCl, 1 mol/L Urea, 10 mmol/L CaCl2 • 2H2O, 0.1 mol/L Citric acid, 5 g/L BSA, pH; 8.0).30 Samples were shaken for 30 min and subsequently centrifuged for 20 min (10.000 rpm, 4 °C). Supernatants were used for analysis of calprotectin using a standard ELISA Kit (HBT) according to the manufacturer’s instructions. Data were expressed as μg/g feces.
Statistical analysisStatistical analyses were performed using SPSS version 20.0. Data were tested for normality by the Kolmogorov-Smirnoff test. Subsequently, continuous variables were presented as median (range) and compared between groups using the Mann-Whitney U test for non-parametric data. Dichotomous variables were compared with the χ2 test. A P <0.05 was considered statistically significant using a two-tailed test.
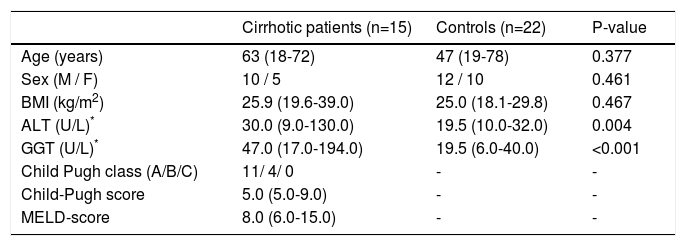
ResultsPatientsDuodenal and/or sigmoid biopsies were obtained from 15 compensated cirrhotic patients (i.e. 12 duodenal and 13 sigmoid biopsies) and from 22 healthy controls (i.e. 22 duodenal and 22 sigmoid biopsies). Characteristics of subjects are given in Table 2. No significant differences with regard to age, sex or BMI were observed between cirrhotic patients and controls. Serum alanine transaminase (ALT) and Gamma-glutamyltranspeptidase (GGT) levels were significantly higher in cirrhotic patients compared to controls (P = 0.004 and P < 0.001, respectively). Furthermore, 11 and 4 patients were classified as Child-Pugh class A and B, respectively. None of the patients had clinically evident complications, i.e. ascites, variceal hemorrhage, hepatic encephalopathy and/or jaundice. Cause of liver cirrhosis was alcohol-related in 4 patients, autoimmune-related in 3 patients, cryptogenic in 4 patients, chronic viral infection in 1 patient, hereditary hemochromatosis in 1 patient and multifactorial in 2 patients.
Characteristics of subjects.
| Cirrhotic patients (n=15) | Controls (n=22) | P-value | |
|---|---|---|---|
| Age (years) | 63 (18-72) | 47 (19-78) | 0.377 |
| Sex (M / F) | 10 / 5 | 12 / 10 | 0.461 |
| BMI (kg/m2) | 25.9 (19.6-39.0) | 25.0 (18.1-29.8) | 0.467 |
| ALT (U/L)* | 30.0 (9.0-130.0) | 19.5 (10.0-32.0) | 0.004 |
| GGT (U/L)* | 47.0 (17.0-194.0) | 19.5 (6.0-40.0) | <0.001 |
| Child Pugh class (A/B/C) | 11/ 4/ 0 | - | - |
| Child-Pugh score | 5.0 (5.0-9.0) | - | - |
| MELD-score | 8.0 (6.0-15.0) | - | - |
M: male. F: female. BMI: body mass Index. ALT: alanine transaminase. U/L: units per liter. GGT: gamma-glutamyltranspeptidase. MELD-score: Model for End-Stage Liver Disease-score. Continuous values are presented as medians (range).
Drug therapy as part of the standard medical care was given to all patients, including among others glucocorticosteroids/immunosuppressives (n = 2), non-steroid antiinflammatory drugs (NSAIDs) (n = 1) and colchicine (n = 1).
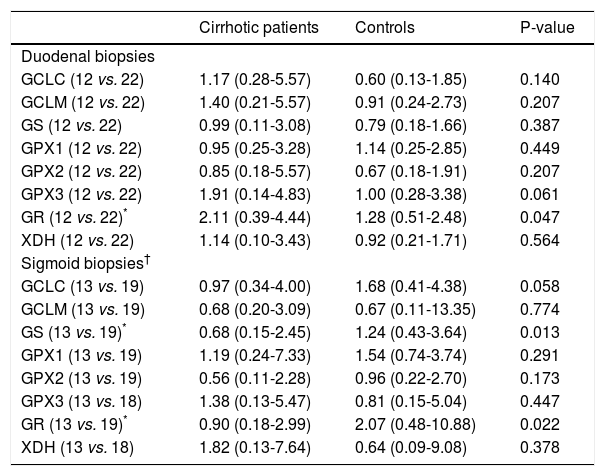
Transcription of oxidative stress-related genesGene transcription of GR was significantly up-regulated in duodenal biopsies of cirrhotic patients vs. controls [2.11 (0.39 – 4.44) vs. 1.28 (0.51 – 2.48); P = 0.047], whereas no significant differences were found for GCLC, GCLM, GS, GPX1, GPX2, GPX3 and XDH between both groups (Table 3).
Transcription of genes involved in glutathione and uric acid metabolism.
| Cirrhotic patients | Controls | P-value | |
|---|---|---|---|
| Duodenal biopsies | |||
| GCLC (12 vs. 22) | 1.17 (0.28-5.57) | 0.60 (0.13-1.85) | 0.140 |
| GCLM (12 vs. 22) | 1.40 (0.21-5.57) | 0.91 (0.24-2.73) | 0.207 |
| GS (12 vs. 22) | 0.99 (0.11-3.08) | 0.79 (0.18-1.66) | 0.387 |
| GPX1 (12 vs. 22) | 0.95 (0.25-3.28) | 1.14 (0.25-2.85) | 0.449 |
| GPX2 (12 vs. 22) | 0.85 (0.18-5.57) | 0.67 (0.18-1.91) | 0.207 |
| GPX3 (12 vs. 22) | 1.91 (0.14-4.83) | 1.00 (0.28-3.38) | 0.061 |
| GR (12 vs. 22)* | 2.11 (0.39-4.44) | 1.28 (0.51-2.48) | 0.047 |
| XDH (12 vs. 22) | 1.14 (0.10-3.43) | 0.92 (0.21-1.71) | 0.564 |
| Sigmoid biopsies† | |||
| GCLC (13 vs. 19) | 0.97 (0.34-4.00) | 1.68 (0.41-4.38) | 0.058 |
| GCLM (13 vs. 19) | 0.68 (0.20-3.09) | 0.67 (0.11-13.35) | 0.774 |
| GS (13 vs. 19)* | 0.68 (0.15-2.45) | 1.24 (0.43-3.64) | 0.013 |
| GPX1 (13 vs. 19) | 1.19 (0.24-7.33) | 1.54 (0.74-3.74) | 0.291 |
| GPX2 (13 vs. 19) | 0.56 (0.11-2.28) | 0.96 (0.22-2.70) | 0.173 |
| GPX3 (13 vs. 18) | 1.38 (0.13-5.47) | 0.81 (0.15-5.04) | 0.447 |
| GR (13 vs. 19)* | 0.90 (0.18-2.99) | 2.07 (0.48-10.88) | 0.022 |
| XDH (13 vs. 18) | 1.82 (0.13-7.64) | 0.64 (0.09-9.08) | 0.378 |
All data are normalized expression ratios, presented as medians (range).
In sigmoid biopsies, gene transcription of both GS and GR was significantly down-regulated in cirrhotic patients compared to controls [0.68 (0.15 – 2.45) vs. 1.24 (0.43 – 3.64); P = 0.013 and 0.90 (0.18 – 2.99) vs. 2.07 (0.48 – 10.88); P = 0.022]. No significant differences in gene transcription were found for GCLC, GCLM, GPX1, GPX2, GPX3 and XDH (Table 3).
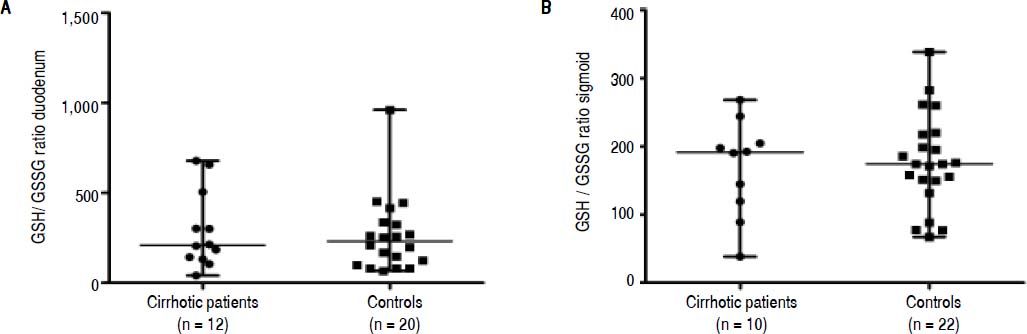
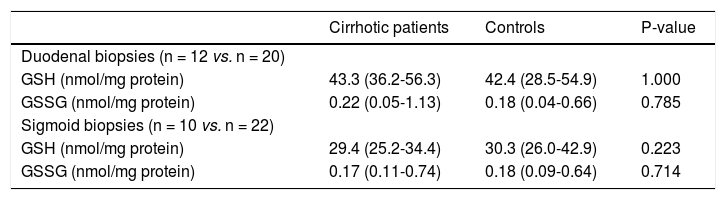
GSH/GSSG ratio and MPO in mucosal biopsiesConcentrations of GSH and GSSG, and the GSH/ GSSG ratio did not differ significantly between cirrhotic patients and controls in duodenal nor in sigmoid biopsies (Table 4 and Figure 1A and 1B).
Glutathione and glutathione disulphide in mucosal biopsies
| Cirrhotic patients | Controls | P-value | |
|---|---|---|---|
| Duodenal biopsies (n = 12 vs. n = 20) | |||
| GSH (nmol/mg protein) | 43.3 (36.2-56.3) | 42.4 (28.5-54.9) | 1.000 |
| GSSG (nmol/mg protein) | 0.22 (0.05-1.13) | 0.18 (0.04-0.66) | 0.785 |
| Sigmoid biopsies (n = 10 vs. n = 22) | |||
| GSH (nmol/mg protein) | 29.4 (25.2-34.4) | 30.3 (26.0-42.9) | 0.223 |
| GSSG (nmol/mg protein) | 0.17 (0.11-0.74) | 0.18 (0.09-0.64) | 0.714 |
All values are medians (range). GSH: glutathione. GSSG: glutathione disulphide.
Glutathione (GSH) / glutathione disulphide (GSSG) ratio in mucosal biopsies of cirrhotic patients and controls. A. Glutathione (GSH) /glutathione disulphide (GSSG) ratio in duodenal biopsies. B. Glutathione (GSH) /glutathione disulphide (GSSG) ratio in sigmoid biopsies. Data are presented as scatter dot plots displaying median with range (P > 0.05).
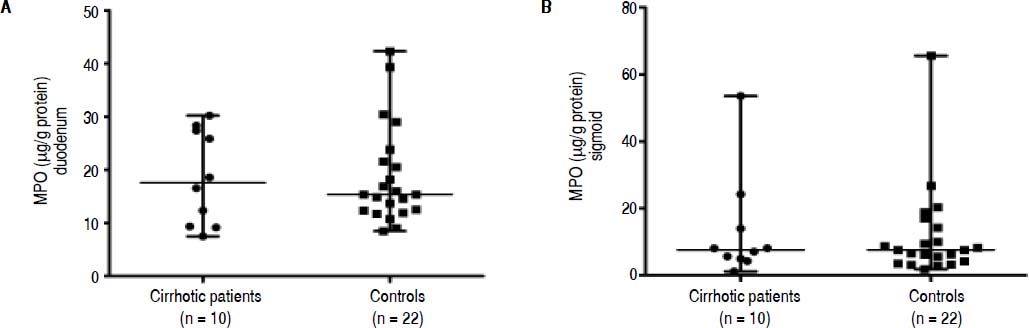
To further support the oxidative stress data, local intestinal inflammation was assessed by analysis of MPO levels in the mucosal biopsies.31 The concentrations of MPO were also not significantly different between both groups in duodenal [17.57 (7.51-30.21) μg/g protein vs. 15.35 (8.48-42.32) μg/g protein; P = 0.968; Figure 2A] nor in sigmoid biopsies [7.56 (1.16-53.55) μg/g protein vs. 7.61 (1.87-65.57) μg/g protein; P = 1.000; Figure 2B].
Myeloperoxidase (MPO) concentrations in mucosal biopsies of cirrhotic patients and controls. A. Myeloperoxidase (MPO) concentrations in duodenal biopsies. B. Myeloperoxidase (MPO) concentrations in sigmoid biopsies. Data are presented as scatter dot plots displaying median with range (P> 0.05).
The intestinal inflammation marker fecal calprotectin did also not differ significantly between cirrhotic patients (n = 15) and controls (n = 22) [(6.37 (0.08-23.46) μg/g feces vs. 1.95 (0.13-29.63) μg/g feces; P = 0.216)].
Histology of mucosal biopsiesMicroscopically only minor morphological changes were observed in duodenal biopsies of cirrhotic patients and controls and did not differ significantly between the groups, such as a (slightly) increased number of intra-epithelial lymphocytes (n = 2 vs. n = 2, respectively), mild edema of the lamina propria (n = 2 vs. n = 3, respectively) and mild chronic inflammation (n = 3 vs. n = 2, respectively). None of the subjects had villous atrophy or signs of acute inflammation. In sigmoid biopsies, only mild edema of the lamina propria was observed in both groups (n = 4 vs. n = 4, respectively).
DiscussionWe investigated the occurrence of oxidative stress in both duodenal and sigmoid mucosal biopsies of patients with compensated liver cirrhosis. Differences were only found for gene transcription of GS and GR in duodenal and sigmoid biopsies between compensated cirrhotic patients and healthy controls, but no alterations were found for other genes nor for protein levels of GSH, GSSG and GSH/GSSG ratio. The intestinal inflammation markers MPO and (fecal) calprotectin were also not different between both groups.
The present study was initiated to investigate whether intestinal oxidative stress occurs in patients with compensated liver cirrhosis and thereby could be an underlying mechanism contributing to the epithelial barrier dysfunction observed in our previous study,28 which potentially may increase the risk of progression towards decompensated cirrhosis.
The transcription of genes involved in the metabolism of GSH and uric acid, which are both important antioxidants and protect cells against oxidative damage, were analyzed. GSH reduces hydrogen peroxide and lipid hydroperoxide to less reactive oxygen species (ROS), and in this process GSH is converted into GSSG. Uric acid is produced from hypoxanthine and xanthine by the enzymes XO and XDH. In vitro, uric acid is a powerful scavenger of ROS32 and by being preferentially oxidized as a substrate for oxidation by haem protein or H2O2 systems, it can protect against oxidative damage.33 As marker of oxidative stress, the GSH/GSSG ratio was determined in both duodenal and sigmoid biopsies.
In the duodenal mucosa, we observed that the gene transcription of GR, the enzyme that recycles GSSG into GSH, was up-regulated in cirrhotic patients. Protein levels of GSH, GSSG and the GSH/GSSG ratio were not altered. These findings may point to an enhancement of repair processes for oxidative stress. In the sigmoid mucosa, gene transcription of GR as well as GS, the latter being one of the enzymes involved in the synthesis of GSH, was down-regulated in cirrhotic patients, which might indicate a decreased formation of GSH. However, protein levels of GSH, GSSG and the GSH/GSSG ratio were not altered. It should be noted that the differences in gene transcription in both duodenum and sigmoid did not remain statistically significant after correcting for multiple testing and therefore should be interpreted with care. In addition, no alterations were found for the other genes investigated. Thereby the above findings indicate that there is no clear evidence for oxidative stress in the intestine of patients with compensated liver cirrhosis. This is further supported by lack of differences in intestinal MPO and fecal calprotectin concentrations between cirrhotic patients and controls. Moreover, we observed no clear morphological abnormalities in the duodenal and sigmoid biopsies.
The lack of changes in oxidative stress and inflammatory parameters is not likely to be attributed to drug therapy as patients used almost no drugs with antioxidant and/or anti-inflammatory effects and no antioxidant supplements.
Alcohol is an important cause of liver cirrhosis and can disturb intestinal epithelial barrier function by inducing intestinal oxidative damage.16,34 In our previous study, small intestinal permeability was found to be increased in a subgroup of patients with alcohol-related cirrhosis, but-the small number of patients with alcohol-related cirrhosis (n = 4) in the present study precluded us from performing additional analyses to investigate whether this subgroup may also be more susceptible for intestinal oxidative stress.
In contrast to the present findings in humans with compensated liver cirrhosis, some animal studies did provide evidence of oxidative stress in the small and large intestine of rats with carbon tetrachloride (CCl4)-induced liver cirrhosis, although part of them had ascites.13,24–26 Published data on the occurrence of intestinal oxidative stress in cirrhotic patients are scarce. Only one other recent study has investigated intestinal oxidative stress in duodenal biopsies of cirrhotic patients by measuring lipid hydroperoxides and found no difference in the levels of lipid hydroperoxides between patients with compensated cirrhosis and healthy controls.27 However, the investigators did find significantly increased levels of lipid hydroperoxides in patients with decompensated cirrhosis when compared to those with compensated cirrhosis and healthy controls. Therefore, we cannot exclude that intestinal oxidative stress does occur in patients with decompensated cirrhosis.
The strength of our study is that we investigated the occurrence of oxidative stress not only in the mucosa of the small, but also of the large intestine in patients with compensated cirrhosis. As we previously did find a clear increased large intestinal permeability, it would be interesting to investigate also other potential causative factors contributing to barrier dysfunction, such as changes in microbiota composition.
Some limitations of the present study should also be taken into account.The GSH/GSSG ratio was analyzed as this ratio is a marker of oxidative stress and gives insight into the body’s anti-oxidant defense capacity.35,36 Because of the limited number of intestinal biopsies available per subject, we were not able to measure other markers of oxidative stress, such as MDA and 4-hydroxynonenal (4-HNE). Our findings are supported by unaltered inflammatory parameters and intestinal morphology. Although we cannot completely exclude the presence of intestinal oxidative stress, the current findings indicate that oxidative stress seems not to be a major factor in these patients. Furthermore, the intestinal biopsies could only be obtained from a rather small group of patients due to the invasiveness of sampling and the complexity of the patient population. Therefore, our results are preliminary and subgroup analyses of cirrhotic patients for example with regard to etiology or drug therapy were not possible. Although we cannot exclude the effect of etiology or drug therapy, nor the possibility that other markers of oxidative stress would be increased in cirrhotic patients, we believe that these factors are not important confounders in this study. Furthermore, inclusion of a larger group of patients is not very likely to change our results as no trend to significance was observed in both oxidative stress and inflammatory parameters between cirrhotic patients and controls.
In conclusion, in stable compensated cirrhosis, there were no indications for the occurrence of oxidative stress and low-grade inflammation in both the small and large intestine. Although these preliminary findings need further validation, we found intestinal oxidative stress not to be a major mechanism contributing to epithelial barrier dysfunction observed in patients with compensated cirrhosis.
Abbreviations- •
4-HNE: 4-hydroxynonenal.
- •
AJ: adherens junction.
- •
ALT: serum alanine transaminase.
- •
CCl4: carbon tetrachloride.
- •
GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
- •
GCLC: glutamate-cysteine ligase, catalytic subunit.
- •
GCLM: glutamate-cysteine ligase, modifier subunit.
- •
GGT: gamma-glutamyltranspeptidase.
- •
GI tract: Gastrointestinal tract.
- •
GPX1, GPX2 and GPX3: glutathione peroxidases 1, 2 and 3.
- •
GR: glutathione reductase.
- •
GS: glutathione synthetase.
- •
GSH: glutathione.
- •
GSSG: glutathione disulphide.
- •
H&E: haematoxylin and eosin.
- •
MDA: malondialdehyde.
- •
MUMC: Maastricht University Medical Center.
- •
NSAIDs: non-steroid anti-inflammatory drugs.
- •
ROS: reactive oxygen species.
- •
TJ: tight junction.
- •
XDH: xanthine dehydrogenase.
- •
XO: xanthine oxidase.