The development of direct-acting antivirals (DAAs) has been a turning point in chronic hepatitis C treatment. With an efficacy rate on viral eradication close to 100% and an excellent safety profile, they have replaced interferon-based treatments as first-line therapy for hepatitis C virus (HCV). Following the encouraging results observed during the first years with these treatments, new publications suggested an unexpectedly high incidence of hepatocellular carcinoma (HCC) in patients previously treated with DAAs as well as a higher HCC recurrence rate in them. The possible interaction between DAAs and HCC and its impact on HCC incidence and recurrence still remains controversial. The aim of the present work is to review the current state of the matter by analyzing studies that evaluate the association between chronic hepatitis C treatment with DAAs and the development of HCC either de novo or as a recurrence. Following this, clinical practice recommendations are done.
HCV chronic infection is a condition that affects approximately 71 million people around the world, 1% of the world’s population, and is one of the major causes of chronic liver disease [1]. Eradication of this viral infection is crucial in order to reduce morbidity and mortality associated with liver cirrhosis and its complications such as portal hypertension and HCC. This neoplasia is the third leading cause of death by cancer worldwide and liver cirrhosis is its most important risk factor [2,3]. In western societies, de novo HCC has an annual incidence rate of 2–8% in cirrhotic patients and HCV is its common etiology.
The development of DAAs has been a turning point in chronic hepatitis C treatment. With an efficacy rate on viral eradication close to 100% and an excellent safety profile, they have replaced interferon-based treatments as first-line therapy for HCV. Furthermore, SVR achieved with these drugs has been associated with an improvement on liver fibrosis measured using transient elastography. Also, it has proven to recover liver function and reduce portal hypertension. Thus, it may even reduce mortality [4–7].
Following the encouraging results observed during the first years with these treatments, new publications suggested an unexpectedly high incidence of HCC in patients previously treated with DAAs as well as a higher HCC recurrence rate in them [8,9]. The possible interaction between DAAs and HCC and its impact on HCC incidence and recurrence still remains controversial. Currently, solid conclusions have not yet been established.
2Material and methodsThe aim of the present work is to review the current state of the matter by analyzing studies that evaluate the association between chronic hepatitis C treatment with DAAs and the development of HCC either de novo or as a recurrence.
Original published studies were identified by searching PubMed database from January 1, 2010 through February 1, 2020 (Fig. 1). English and Spanish language articles were selected using the following keywords: hepatocellular carcinoma, chronic hepatitis C and direct-acting antivirals. Prospective or retrospective cohort studies, reviews, and guidelines with experience on human treatment were included. Journals with low impact factor were not considered. A full-text evaluation was performed, and references from relevant articles were reviewed to identify additional studies.
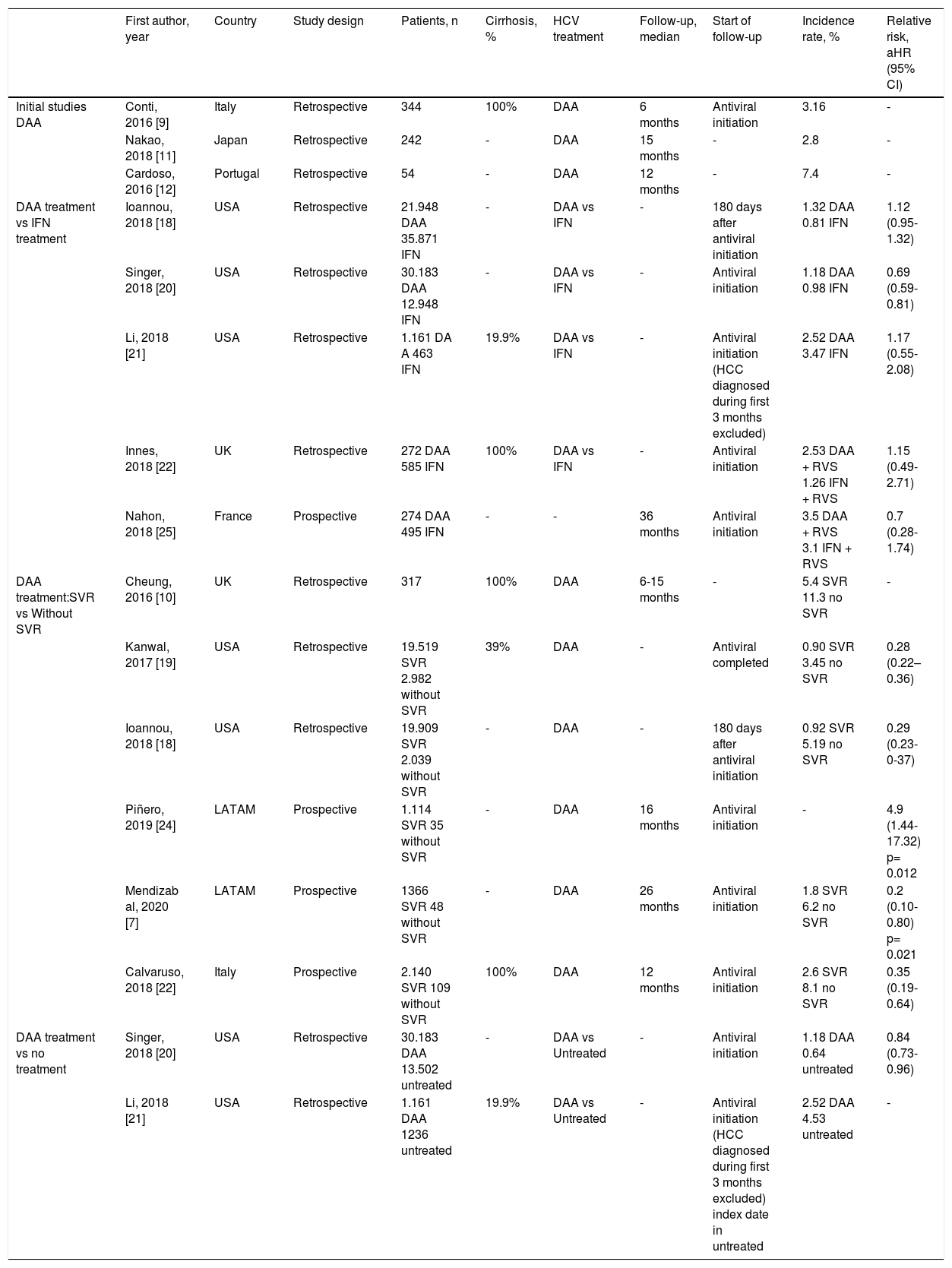
3Results3.1Incidence of HCC after DAA-therapySome years ago, several studies surprisingly suggested that HCC could be earlier developed, or its incidence increased in patients with HCV-induced liver cirrhosis and treated with DAAs. In 2016, in a cohort of HCV-cirrhotic patients treated with DAAs Conti et al. observed that there was not a reduction on HCC incidence [9]. In this study, 9 out of 285 patients (3.16%; 95% CI, 1.45–5.90) developed an HCC on the 24 weeks following therapy and the 1-year cumulative incidence was 3.2%. Although no control group was used, the incidence of HCC was like to the one that had been observed in a historical cohort of non-treated HCV-cirrhotic patients from the same geographical area. A similar study pointed out that HCC incidence at six-month time was the same for the group treated with DAAs (17/406) and for the non-treated group (11/261), both of 4%, suggesting that DAAs therapies did not reduce the incidence of HCC [10]. Afterwards, Nakao et al. described similar findings regarding HCC frequency and noticed less differentiated tumors with a higher growing rate in patients that had received DAA treatment [11]. Furthermore, Cardoso et al. observed a higher incidence of HCC after DAAs treatment [12]. One year after achieving viral suppression, HCC was diagnosed in 7.4% of DAA therapy patients at a median time of 7.6 months (IQR 6.3–10.6 months) in comparison to the one seen after IFN treatment (1.2–1.4%). The results of these studies with DAAs contrast sharply with the ones observed in two meta-analysis on IFN-based treatments. They demonstrated a 65-74% decrease in HCC incidence on patients with SVR compared to patients without response (RR 0.35; 95% CI, 0.26 – 0.46) [13] vs (RR 0.24; 95% CI, 0.18−0.31) [14]. Different hypothesis were formulated to explain higher incidence of HCC in patients treated with DAAs. It was suggested that immune system surveillance could have been altered as a consequence of fast HCV replication inhibition by DAAs, leading to an escape of tumor cells [15]. Also, DAA based therapy might modify liver carcinogenesis, which would not follow a multistep process. Some mechanisms potentially involved in it could be an increment of serum vascular endothelial growth factor levels and a reduction of natural killer group 2 member D. The first of these mechanisms is associated to tumor angiogenesis [16] and the second is correlated to the control of cancer by the immune system [17].
Subsequent studies with a wide number of patients, some national registries and prospective cohort studies of patients under surveillance for early HCC diagnosis, showed results that conflicted the one’s seen before, as they described a reduction on the incidence of HCC after DAA therapy. One of which was a retrospective study of the US Department of Veteran Affairs with 62.000 patients that correlated SVR to a 71% decrement on HCC, without differences on the groups of treatment with DAA or IFN [DAA-only (aHR 0.29; 95% CI, 0.23–0.37], DAA + IFN (aHR 0.48; 95% CI, 0.32–0.73) or IFN-only (aHR 0.32; 95% CI, 0.28–0.37)] [18]. Another cohort of 22.500 patients showed a 76% reduction on HCC in patients with RVS compared to patients without VRS (0.90 vs 3.45 cases/100 persons-year [aHR 0.28, 95% CI, 0.22−0.36]) [19]. This study concluded that the risk of developing HCC did not disappear completely even though it was clear that it was reduced. This risk was higher in cirrhotic patients (ranging from 1.0 to 2.2% per year, depending on epidemiological and clinical characteristics of the patients). Further studies confirmed this decrease on the probability of developing HCC de novo after DAA-therapy (Table 1) [20–23].
HCC incidence after DAA-therapy.
| First author, year | Country | Study design | Patients, n | Cirrhosis, % | HCV treatment | Follow-up, median | Start of follow-up | Incidence rate, % | Relative risk, aHR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Initial studies DAA | Conti, 2016 [9] | Italy | Retrospective | 344 | 100% | DAA | 6 months | Antiviral initiation | 3.16 | - |
| Nakao, 2018 [11] | Japan | Retrospective | 242 | - | DAA | 15 months | - | 2.8 | - | |
| Cardoso, 2016 [12] | Portugal | Retrospective | 54 | - | DAA | 12 months | - | 7.4 | - | |
| DAA treatment vs IFN treatment | Ioannou, 2018 [18] | USA | Retrospective | 21.948 DAA 35.871 IFN | - | DAA vs IFN | - | 180 days after antiviral initiation | 1.32 DAA 0.81 IFN | 1.12 (0.95-1.32) |
| Singer, 2018 [20] | USA | Retrospective | 30.183 DAA 12.948 IFN | - | DAA vs IFN | - | Antiviral initiation | 1.18 DAA 0.98 IFN | 0.69 (0.59-0.81) | |
| Li, 2018 [21] | USA | Retrospective | 1.161 DA A 463 IFN | 19.9% | DAA vs IFN | - | Antiviral initiation (HCC diagnosed during first 3 months excluded) | 2.52 DAA 3.47 IFN | 1.17 (0.55-2.08) | |
| Innes, 2018 [22] | UK | Retrospective | 272 DAA 585 IFN | 100% | DAA vs IFN | - | Antiviral initiation | 2.53 DAA + RVS 1.26 IFN + RVS | 1.15 (0.49-2.71) | |
| Nahon, 2018 [25] | France | Prospective | 274 DAA 495 IFN | - | - | 36 months | Antiviral initiation | 3.5 DAA + RVS 3.1 IFN + RVS | 0.7 (0.28-1.74) | |
| DAA treatment:SVR vs Without SVR | Cheung, 2016 [10] | UK | Retrospective | 317 | 100% | DAA | 6-15 months | - | 5.4 SVR 11.3 no SVR | - |
| Kanwal, 2017 [19] | USA | Retrospective | 19.519 SVR 2.982 without SVR | 39% | DAA | - | Antiviral completed | 0.90 SVR 3.45 no SVR | 0.28 (0.22–0.36) | |
| Ioannou, 2018 [18] | USA | Retrospective | 19.909 SVR 2.039 without SVR | - | DAA | - | 180 days after antiviral initiation | 0.92 SVR 5.19 no SVR | 0.29 (0.23-0-37) | |
| Piñero, 2019 [24] | LATAM | Prospective | 1.114 SVR 35 without SVR | - | DAA | 16 months | Antiviral initiation | - | 4.9 (1.44-17.32) p= 0.012 | |
| Mendizab al, 2020 [7] | LATAM | Prospective | 1366 SVR 48 without SVR | - | DAA | 26 months | Antiviral initiation | 1.8 SVR 6.2 no SVR | 0.2 (0.10-0.80) p= 0.021 | |
| Calvaruso, 2018 [22] | Italy | Prospective | 2.140 SVR 109 without SVR | 100% | DAA | 12 months | Antiviral initiation | 2.6 SVR 8.1 no SVR | 0.35 (0.19-0.64) | |
| DAA treatment vs no treatment | Singer, 2018 [20] | USA | Retrospective | 30.183 DAA 13.502 untreated | - | DAA vs Untreated | - | Antiviral initiation | 1.18 DAA 0.64 untreated | 0.84 (0.73-0.96) |
| Li, 2018 [21] | USA | Retrospective | 1.161 DAA 1236 untreated | 19.9% | DAA vs Untreated | - | Antiviral initiation (HCC diagnosed during first 3 months excluded) index date in untreated | 2.52 DAA 4.53 untreated | - |
Using the US administrative claims database Singer et al. concluded that patients under DAA therapy had a lower frequency of HCC in comparison to those non treated (aHR 0.84, 95% CI, 0.73−0.96) and to the controls in pre-DAAs times with IFN-based treatment (HR 0.69, 95% CI, 0.59−0.81) [20]. On a national retrospective cohort, no differences were found on HCC incidence among patients treated with IFN or those treated with DAAs (HR 1.07; 95% CI, 0.55–2.08). Furthermore, it concluded that non-treated cirrhotic patients had a higher incidence rate of HCC (4.53 per 100 persons-years) than those that had received IFN or DAAs (3.47 and 2.51 per 100 persons-years, respectively [p = 0.03]). Calvaruso et al. observed a lower incidence of HCC in patients treated with DAA and noticed that these patients generally developed smaller unicentric tumors [23].
Piñero et al. published a prospective multicenter cohort study that included 1400 F1-F4 patients treated with DAAs in Latin America. The median follow-up was 16 months from the start of DAAs treatment. It concluded that achieving SVR with DAAs regimens was associated with an overall reduction of the relative risk for the novo HCC of 73%, with a cumulative incidence of HCC 0.02 (95% CI, 0.1−0.3) at 12 months and 0.04 (95% CI, 0.3−0.6) at 24 months. Failure to achieve SVR was independently associated to development of de novo HCC with a HR of 4.9 (95% CI, 1.44−17-32). A sensitivity analysis that included cirrhotic patients (n = 784) showed that cumulative incidence of HCC at 12 months was 0.03 (95% CI, 0.02‐0.05) and 0.06 (95% CI, 0.04‐0.08) at 24 months of follow‐up respectively [24]. Recently, Mendizabal et al. carried out in the same geographical area a larger cohort multicenter study. It was a prospective study of 1760 F0-F4 patients who had no history of decompensation and that had received DDA treatment [7].The authors demonstrated a clear reduction in the risk of new liver-related complications. SVR significantly reduced the incidence of HCC (HR 0.2; 95% CI, 0.1−0.8%, p = 0.02) in the overall cohort. Additionally, a relevant discovery was made when it was observed that patients with F0 to F2 liver fibrosis and SVR were still at risk of developing HCC (HR 0.08; 95% CI, 0.01−0.8%, p = 0.036).
A prospective French cohort of patients with HCV-related cirrhosis compared the incidence of HCC among those treated with DAAs and IFN [25]. The results showed a higher frequency of HCC in patients treated with DAAs in comparison to those treated with IFN after 3 years: 5.9% in the DAA group, 3.1% in the SVR-IFN group, and 12.7% in the non-SVR group (p < 0.001; unadjusted HR for HCC 2.03; 95% CI, 1.07–3.84; p = 0.030 for the DAA group vs the SVR-IFN group). A detailed analysis of these results suggested this could be explained by the fact that patients treated with DAAs had older age, diabetes, and a worst liver function, three elements that are risk factors for HCC. Further analysis was made with this elements as confounding factors and the risk of HCC found was not higher in patients that received DAA (HR 0.89; 95% CI, 0.46–1.73; p = 0.73).
However, a recent study run by Mariño et al. [26] with 1123 patients during a 19.3 months follow-up period described a 3.73/100 people-year (95% CI, 2.96–4.70) incidence of HCC, with a median time to development of 10.3 months from the start of DAA treatment. This study revealed a great increase on the relative risk of developing HCC in patients with liver nodules “non-characterized” before DAA therapy, RR 2.83 (95% CI, 1.55–5.16). These results could indicate a temporal association between antiviral agents and the development of HCC as well as an augmented risk for HCC in patients that had undetermined nodules before starting DAAs. The authors suggest that the results might be due to DAAs interfering on immune surveillance mechanisms leading to dysplastic cells or clone activation that would result on a higher risk of HCC in the short term. Another possible explanation to this is based on the fact that nowadays more severe cases of HCV can be treated, and some may even have advanced liver cirrhosis with severe impairment of its function. Therefore, these patients have an intrinsically increased risk of developing HCC. However, it can not be clearly concluded that there was a higher risk of HCC as of the patients included were treated with DAAs and there was no control group. In addition to this, making comparisons with patients eligible for IFN-based therapy from historical cohorts can result in a selection bias as criteria to initiate IFN-based treatment excludes the most severe patients (Child-Pugh B or C) unlike the ones who received DAAs on the present report. Furthermore, the increased short-term liver cancer risk described in this study could be explained by the fact that the average follow-up time post-initiation therapy was shorter in this study (9.8 months) than the one in IFN cohorts (EPIC: 31.4 months; HALT-C: 42.0 months).
Currently there is no explanation for a potential increase of the novo HCC in patients treated with DAAs. Direct oncological effect of DAAs is unlikely. Several researchers have proposed that DAAs induce a breakdown of immune surveillance [14]. One tentative explanation is that cancer emergence would be facilitated by changes in the immune response (hyporesponsive state of memory-helper T cells, etc) and a decrease of the anti-inflammatory reaction, considering both as consequences of the quick viral clearance.
3.2HCC recurrence after DAA-treatmentHCV eradication with IFN-based treatment in HCC patients had demonstrated a decrease in tumor recurrence and an improvement in survival. Along these lines, a systematic review and a meta-analysis showed the benefit on recurrence [OR 0.19 (0.06−0.60); p = 0.005] and survival [OR 0.31 (0.11−0.90); p = 0.03] in patients with SVR [27].
In 2016, two studies in Spain and Italy unexpectedly concluded that there was a higher HCC recurrence rate and an early development of it in patients that had received DAA. These were small studies which did not have a control group [8,9]. Although both studies were retrospective cohorts of a limited number of patients, its findings were shocking as they showed an early and higher rate of tumor recurrence than expected. Its authors hypothesized that the fast decrease on viral load caused by antiviral treatment would reduce certain inflammatory responses causing the suppression of the immune process and the growth of latent tumor clone cells [4,28,29].
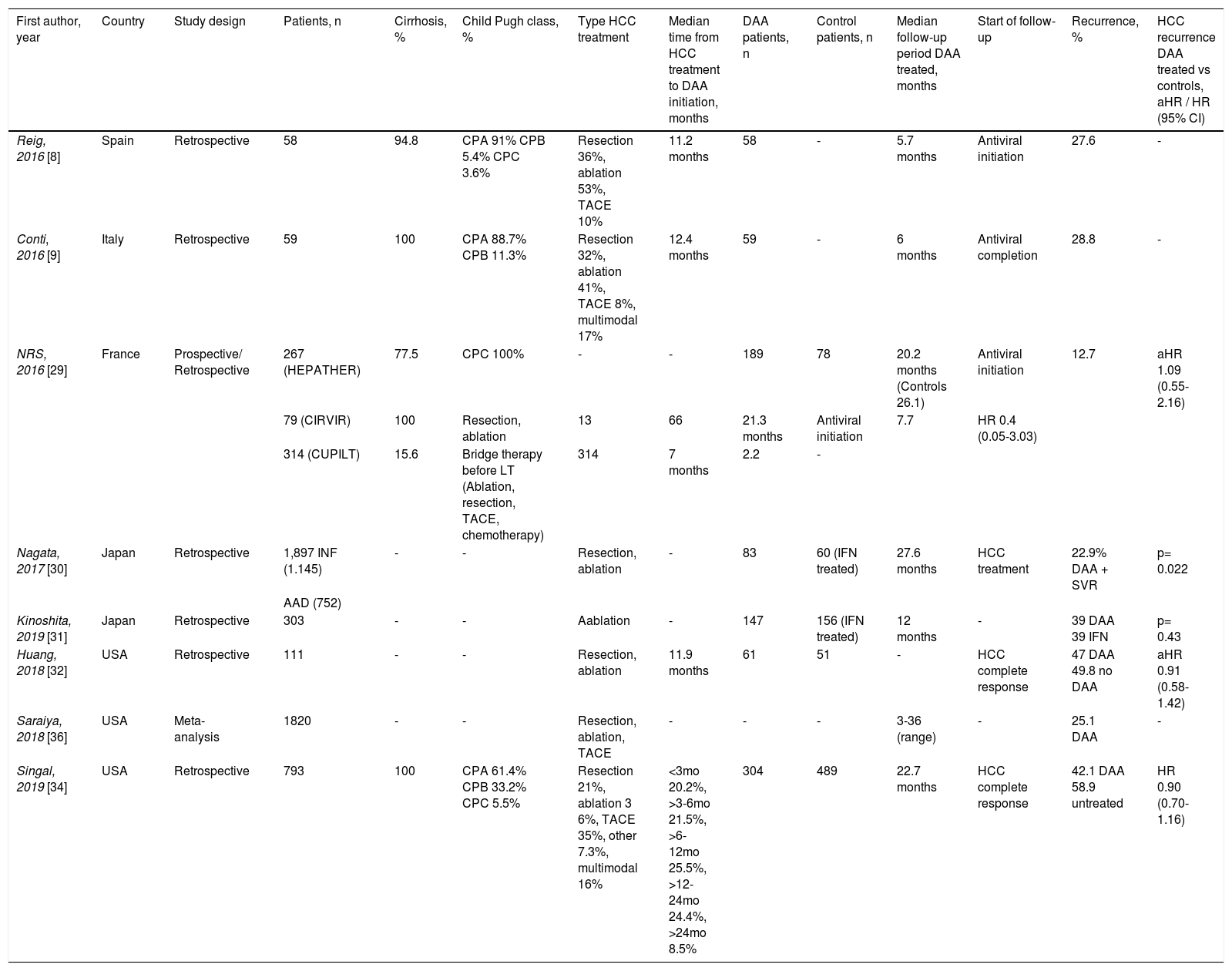
The multicenter study carried out by Reig et al. in Spain, described tumor recurrence on 16 (27.6%) out of 58 patients in a median follow-up time of 5.7 months from the start of antiviral treatment. This rate of HCC recurrence in patients with low or moderate theoretical risk of it could suggest an association with DAA therapy used for HCV eradication. In order to sense determinate if the recurrence rate is higher than expected, the authors scrutinized several data sources. One of them was the double-blind placebo-controlled STORM trial, that tested the efficacy of sorafenib in preventing recurrence after surgical resection or ablation. STORM did not include patients with solitary HCC < 2 cm if pathology did not disclose characteristics linked to high recurrence risk. Furthermore, since the follow-up period of treated patients was not as long as in the STORM trial, the authors specifically assessed the probability of recurrence within the first 4 months of achieving the complete response. The recurrence rate in the analyzed datasets was consistently lower than the figures observed in DAA treated patients. In the subgroup of 17 patients with a limited time (<4 months) between HCC treatment, complete response and DAA treatment initiation (similar population to STORM) the recurrence rate in Reig et al cohort (41.2%) was also higher than the reported in STORM (21.5%). Obviously, the limited number of cases in each stratum, make the comparisons not robust enough and therefore the results should be considered with caution. One of the important methodological limitations of the study is the temporal bias. The median time between total tumor eradication and initiation of anti-HCV therapy was 11.2 months. In other series this time was longer and it could be the explanation to the disparity observed in the results. In fact, a long-time interval before starting DAAs could entail a lower risk of detecting residual tumors.
Further studies have reported contrasting results regarding risk of HCC recurrence (Table 2). In this sense, two French cohorts did not find an increase on recurrence risk after DAA treatment. The recurrence rates found were 7.7% (95% CI, 0.2−0.36) and 12.7% (95% CI, 8.3–18.3) and the multivariate analysis showed no difference in HCC recurrence rates between treated and non-treated patients [30]. The HEPATHER, CirVir and CUPILT cohorts were all characterized by prospective rigorous multicenter protocol-driven systematic data collection and analysis of predefined outcomes. Another strength of this work is the authors’ focus on patients previously treated for HCC using curative procedures (hepatic resection, percutaneous ablation, or liver transplantation) excluding patients treated with chemoembolization. So, these cohorts with an important size and designed with a suitable methodology provide unique information giving consistent results and significant knowledge.
HCC recurrence after DAA-treatment.
| First author, year | Country | Study design | Patients, n | Cirrhosis, % | Child Pugh class, % | Type HCC treatment | Median time from HCC treatment to DAA initiation, months | DAA patients, n | Control patients, n | Median follow-up period DAA treated, months | Start of follow-up | Recurrence, % | HCC recurrence DAA treated vs controls, aHR / HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reig, 2016 [8] | Spain | Retrospective | 58 | 94.8 | CPA 91% CPB 5.4% CPC 3.6% | Resection 36%, ablation 53%, TACE 10% | 11.2 months | 58 | - | 5.7 months | Antiviral initiation | 27.6 | - |
| Conti, 2016 [9] | Italy | Retrospective | 59 | 100 | CPA 88.7% CPB 11.3% | Resection 32%, ablation 41%, TACE 8%, multimodal 17% | 12.4 months | 59 | - | 6 months | Antiviral completion | 28.8 | - |
| NRS, 2016 [29] | France | Prospective/ Retrospective | 267 (HEPATHER) | 77.5 | CPC 100% | - | - | 189 | 78 | 20.2 months (Controls 26.1) | Antiviral initiation | 12.7 | aHR 1.09 (0.55-2.16) |
| 79 (CIRVIR) | 100 | Resection, ablation | 13 | 66 | 21.3 months | Antiviral initiation | 7.7 | HR 0.4 (0.05-3.03) | |||||
| 314 (CUPILT) | 15.6 | Bridge therapy before LT (Ablation, resection, TACE, chemotherapy) | 314 | 7 months | 2.2 | - | |||||||
| Nagata, 2017 [30] | Japan | Retrospective | 1,897 INF (1.145) | - | - | Resection, ablation | - | 83 | 60 (IFN treated) | 27.6 months | HCC treatment | 22.9% DAA + SVR | p= 0.022 |
| AAD (752) | |||||||||||||
| Kinoshita, 2019 [31] | Japan | Retrospective | 303 | - | - | Aablation | - | 147 | 156 (IFN treated) | 12 months | - | 39 DAA 39 IFN | p= 0.43 |
| Huang, 2018 [32] | USA | Retrospective | 111 | - | - | Resection, ablation | 11.9 months | 61 | 51 | - | HCC complete response | 47 DAA 49.8 no DAA | aHR 0.91 (0.58-1.42) |
| Saraiya, 2018 [36] | USA | Meta-analysis | 1820 | - | - | Resection, ablation, TACE | - | - | - | 3-36 (range) | - | 25.1 DAA | - |
| Singal, 2019 [34] | USA | Retrospective | 793 | 100 | CPA 61.4% CPB 33.2% CPC 5.5% | Resection 21%, ablation 3 6%, TACE 35%, other 7.3%, multimodal 16% | <3mo 20.2%, >3-6mo 21.5%, >6-12mo 25.5%, >12-24mo 24.4%, >24mo 8.5% | 304 | 489 | 22.7 months | HCC complete response | 42.1 DAA 58.9 untreated | HR 0.90 (0.70-1.16) |
The review of a prospective Japanese database by Nagata et al. concluded that there was a lower recurrence in patients with SVR after treatment with no difference between patients treated with DAAs and the ones treated with IFN (3-year incidence: 1.4% in IFN-free therapy, 3.3% in IFN-based, p = 0.49) [31]. Additionally, in a cohort of HCC patients treated with radiofrequency Kinoshita et al. described similar recurrence rates in SVR attained by DAAs or by IFN. These recurrence rates were measured at two points in time: 1 year after antiviral therapy and 2 years after (recurrence rates for DAA were 39% and 60%) (recurrence rates for IFN were 39% and 61%) (p = 0.43) [32]. Nevertheless, this study has several limitations. First of all, there was no control arm and the observational period of the DAA group was relatively short. Also, the baseline characteristics of the IFN group and the DAA group were different. Finally, patients treated with DAAs after failure of IFN therapy were included in the IFN group, which could have caused a bias.
Furthermore, Huang et al. analyzed a group of patients on the liver transplant waiting list with pre-locoregional therapy and found a similar probability of developing another HCC in those with DAA-therapy (47%) and those non-treated (49.3%) (HR 0.91; 95% CI, 0.58–1.42, p = 0.67) in a year period. Also, DAA treated patients sustained a lower risk of being removed from the waiting list during the following year because of tumor progression or death (HR 0.30; 95% CI, 0.13−0.69, p = 0.005) [33].
Saraiya et al. conducted a meta-analysis including 24 studies with a total of 1820 patients to analyze the recurrence of HCC after DAA therapy. The recurrence rates at two years’ time after DAA treatment were heterogeneous, ranging from 0 to 59% (pooled estimate 24.4%; 95% CI, 18.4%–30.4%). Nine of the analyzed studies allocated its patients in further groups: treated with IFN and/or non-treated patients. In five of these studies the group treated with DAAs showed no differences on the recurrence of HCC while 2 studies showed a lower recurrence rate on patients that had received DAAs versus the ones that had not (pooled OR 0.55; 95% CI, 0.25−0.85). The results were similar in 3 studies that compared patients that underwent DAA therapy with those treated with IFN [34].
Afterwards, two North American cohort studies with a large number of patients that had been treated with DAAs analyzed the recurrence of HCC. Singal et al. carried out a multicenter retrospective study of 793 patients with HCC that underwent locoregional treatment or liver resection [35]. Recurrence was observed in 128/304 (42.1%, early recurrence in 52 cases) patients treated with DAAs and 288/489 (58.9%, early recurrence in 227 cases) non-treated patients. Neither total recurrence (HR 0.90, 95% CI, 0.70–1.16) nor early recurrence (HR 0.96, 95% CI, 0.70–1.34) were increased in DAA therapy group. However, there are some significant drawbacks in this research. The imaging interpretation used was the one available from routine clinical care instead of being a centralized review, affecting this the classification of HCC complete response. Furthermore, they had a 9% loss of the patients during follow-up period (with a higher proportion in the DAA-untreated group). Also, data on development of hepatic decompensation was not collected during follow-up and they were not able to demonstrate that the survival benefit observed was a direct consequence of liver function preservation.
Besides that, a study by Zou et al. found a recurrence in 26.1% of the patients during a 23.3 months follow-up period. It was observed that patients that had received a non-curative treatment had a higher risk of recurrence than those that received a curative one (aHR 2.06, 95% CI, 1.24–3.40) [36]. Also, it demonstrated that treatment with DAAs was beneficial. In fact, patients that had not achieved SVR showed a higher risk of HCC recurrence (0.8 per 1000 person-month) in comparison to the ones that had SVR (0.35 per 1000 person-month) (aHR 4.17, 95% CI, 1.48–11.75). Another important conclusion drawn was that time from HCC treatment to the start of DAA therapy had an important role in future recurrence of HCC. Recurrence rate was lower when this period of time lasted longer. As the authors could not find an explanation to this, they hypothesized about DAAs’ interactions with the tumor and the immune system. Furthermore, it is possible that in some cases classified as HCC recurrence not enough time was left after treatment to evaluate complete tumor response.
4Discussion4.1Methodological limitationsEstablishing solid conclusions on interaction between DAAs for HCV patients and HCC is difficult due to heterogeneity of results and the restricted quality of the studies, mainly because of their design. These issues have been recently reviewed [34]. Generally speaking, studies on incidence of HCC provide a higher level of evidence than research on recurrence, which has more methodological limitations and biases.
A large part of the investigations done on HCC incidence are retrospective, have a limited size or do not have a control group. It is also frequent that the ones that have a control group compare patients that have received DAAs to IFN. However, this comparison is biased as baseline clinical characteristics of patients treated with IFN differ from the ones treated with DAAs. For instance, DAA therapy can be used in patients with a higher degree of liver dysfunction. Comparison of outcomes in patients treated with DAAs to those left untreated can be limited by confounding factors [5]. Sometimes the selection of patients that undergo a certain treatment is biased as there are reasons, such as co-morbidities, that limit the eligibility of patients for them. In other studies, the outcomes of untreated patients are frequently followed in a different and less careful way than those treated. Also, the immortal time bias is sometimes noticed. This bias occurs when a cohort is followed up a period of time during which death or an outcome that determines end of follow-up cannot occur due to the design of the study. Finally, statistical methodology used in research is heterogeneous. For instance, scarce studies use time-covariate analysis to adjust the effect of baseline variables during follow-up assessment.
Studies on recurrence of HCC in treated patients have important variations regarding the number of previous recurrences, tumor burden (early HCC or not), recurrence risk (multicentricity, microvascular invasion or elevated α-fetoprotein) and type of antitumor therapy (curative or non-curative). Frequently, not all these basal characteristics are described and therefore, cases are not accordingly stratified in the analysis, modifying as a result recurrence rates. This is seen in a great number of studies as they include patients with non-early-stage HCC, patients with past recurrences, and patients who received noncurative locoregional therapies. Moreover, there is not a standard protocol in studies for recurrence detection after tumor treatment, which leads to heterogeneity. Additionally, patients with suspicious liver lesions identified before DAA therapy are frequently not excluded from studies, so cases diagnosed as a recurrence might in fact be a pre-existent tumor. Lastly, there are variations on the moment established for the start of the follow-up period among the studies. This time may be calculated from HCC treatment, from antiviral therapy initiation or from antiviral completion. Depending on each method different recurrence rates are obtained. The time delay between HCC treatment and antiviral initiation also varies widely. Meta-regression analysis pointed out that a short interval between HCC cure and DAA initiation [34] is the most common factor associated with recurrence. This suggests that many cases of “recurrence” are in fact patients who have not achieved a durable complete response.
Although the evidence available on DAA effects on HCC incidence and recurrence is still limited and generates considerable controversy, some conclusions and recommendations could be made in the light of the existing knowledge.
4.2Follow-up of chronic hepatitis C with SVR after DAA-therapy and de novo HCCSVR results in 70–75% decrease on the risk of de novo HCC. These results are seen irrespective to the therapy used: DAAs or IFN. In the beginning, several studies concluded that treatment with DAAs did not reduce the risk of HCC or that they even increased it. But these studies were small, had no control group and they generally had a short follow-up, which complicated obtaining ultimate conclusions. An important number of subsequent studies, with higher methodological quality, confirmed that antiviral treatment has overall a positive effect on HCC incidence.
Existing clinical guidelines on hepatitis C recommend follow-up after attaining SVR in patients that had advanced liver fibrosis (F3/F4) prior to HCV treatment because of the risk of HCC. Further surveillance of cirrhotic patients is also recommended as HCC development risk prevails [37–40]. However, elements such as advanced age, high degree of liver dysfunction, persistence of necroinflammatory activity markers, comorbidity (metabolic syndrome, etc.) and ethanol consumption, can increase the risk of de novo HCC after achieving SVR [41]. It remains unclear whether regression of liver fibrosis could result in a lower risk of liver cancer and therefore less need of surveillance. Another important issue is how fibrosis is quantified during follow-up after viral eradication. There is a lack of studies using sequential histological examination before and after DAA therapy. Currently fibrosis is evaluated with elastography or serum biomarkers, but the reliability of both techniques has not yet been standardized or fully confirmed for this context. There is evidence that cirrhotic patients with persistent high values of FIB-4/APRI after attaining SVR have a higher risk of developing HCC. In conclusion, ultrasound follow-up every six months is recommended for cirrhotic patients with SVR. Although there still is no solid evidence, it is possible that if a liver biopsy confirms cirrhosis reversion to a less advanced state of fibrosis, further follow-up might not be necessary.
There is not much information available on the risk of developing HCC after HCV eradication on patients with F3 fibrosis stage before treatment. Current data suggests that this risk is low (0.16−0.34% annually), which would mean that ultrasound surveillance is not a cost-effective strategy [5,42,43]. Therefore, it would be necessary to evaluate the individual risk of each subject. Another concern is that there is not a clear limit between F3 and F4 stages of liver cirrhosis on non-invasive diagnostic techniques. Guidelines on HCC from different scientific societies make different recommendations [39,43,44]. In this manner, EASL (European Association for the Study of the Liver) and ESMO (European Society for Medical Oncology) suggest follow-up in F3 fibrosis patients while AASLD (American Association for Study of Liver Diseases) states that no further procedure is required. In consequence, until further evidence, a surveillance strategy such as the one followed by cirrhotic patients (an ultrasound biannually) could be suggested for patients with F3 fibrosis.
It has been suggested that once SVR is confirmed, patients with non-significant liver fibrosis (F0-F1) could be discharged without further follow-up [41]. There is limited evidence on the risk of HCC in patients with moderate fibrosis (F2). However, a recent study has shown that even patients with fibrosis grade ranging from 0 to 2 are at risk of developing HCC [7]. Therefore, more studies are needed to clarify if F0-F2 patients’ risk of HCC reaches the cut-off point (0.8-1.5% per year) beyond which HCC surveillance becomes cost-effective [19].
Ultrasound is the most widely used test for surveillance as serological tests are not accurate enough for routine surveillance of early HCC [44]. Some studies have shown a benefit in overall survival with the combination of US and alpha-fetoprotein (AFP) [45,46]. Yet, there is no data available to include AFP as part of the surveillance strategy in patients previously treated with DAAs.
4.3Recurrence and surveillance of patients with HCC history and SVR after treatment with DAATwo initial studies showed a high HCC recurrence rate and some cases of more aggressive HCC after DAA therapy. However, these publications and others alike had methodological limitations. Subsequent research obtained different conclusions, indicating less or at least similar risk of HCC recurrence. Current available data does not enable to make ultimate recommendations. It might be reasonable to delay DAA therapy up to 6 months after complete response is evidenced by contrast-enhanced MRI or CT. Complete response should be evaluated by undergoing two times either of the tests, separated each of them by a three month period [40,41,45]. In addition to this, an adequate follow-up on patients with SVR is necessary as recurrence risk prevails on them. A contrast-enhanced CT or MRI every 3 months during the first year and twice a year afterwards is a surveillance strategy recommended [41,46–48]. No data suggests that follow-up intervals should be shortened and it still remains unclear when ultrasound surveillance should be reintroduced or whether MRI or CT should be indefinitely maintained [41].
5ConclusionsThis narrative review updates the evidence describing the impact of DAA therapy on HCC, measured as the risks of occurrence and recurrence. The studies done on this effect have methodological limitations and offer controversial results.
Available studies show that using DAAs as the treatment for patients with HCV infection is effective on primary prevention of HCC. Several large cohort studies have proved that DAA-induced SVR reduces the risk for de novo HCC. So treating HCV is a good approach to prevent this neoplasia. However, the residual risk for HCC after achieving SVR justifies surveillance of patients with advanced fibrosis.
Controversy on HCC recurrence continues and robust data to support it is still missing. Further higher-quality studies are needed to determine the consequences of DAA therapy on the risk of HCC reappearance and its aggressiveness in patients that previously underwent HCC treatment. It might be reasonable to use DAA therapy after HCC treatment but delaying its start at least 6 months after complete response is confirmed.
Conflict of interestThe authors have no conflicts of interest to declare.