Interferon-based simeprevir therapy showed high efficacy and tolerability in children with genotype 1 hepatitis C virus infection. While direct-acting antivirals (DAAs) therapy are undergoing study in children, this regimen is considered an available therapeutic option for selected patients in countries where DAAs have not yet been approved.
Antiviral therapy in patients with hepatitis C virus (HCV) infection has entered a new era with the development of direct-acting antivirals (DAAs). Some clinical trials of DAAs to improve tolerability and compliance in children are ongoing, and the US Food and Drug Administration (FDA) first approved two hepatitis C drugs for pediatric patients 12 years of age and older in April 2017.1 However, since DAA therapy is not affordable, interferon-based (IFN-based) regimens may still be appropriate, especially in developing countries. Up to now, reports of studies of IFN-based simeprevir (SMV) therapy for pediatric patients have been limited. Thus, six pediatric cases with genotype 1 HCV infections treated by IFN-based SMV therapy are described.
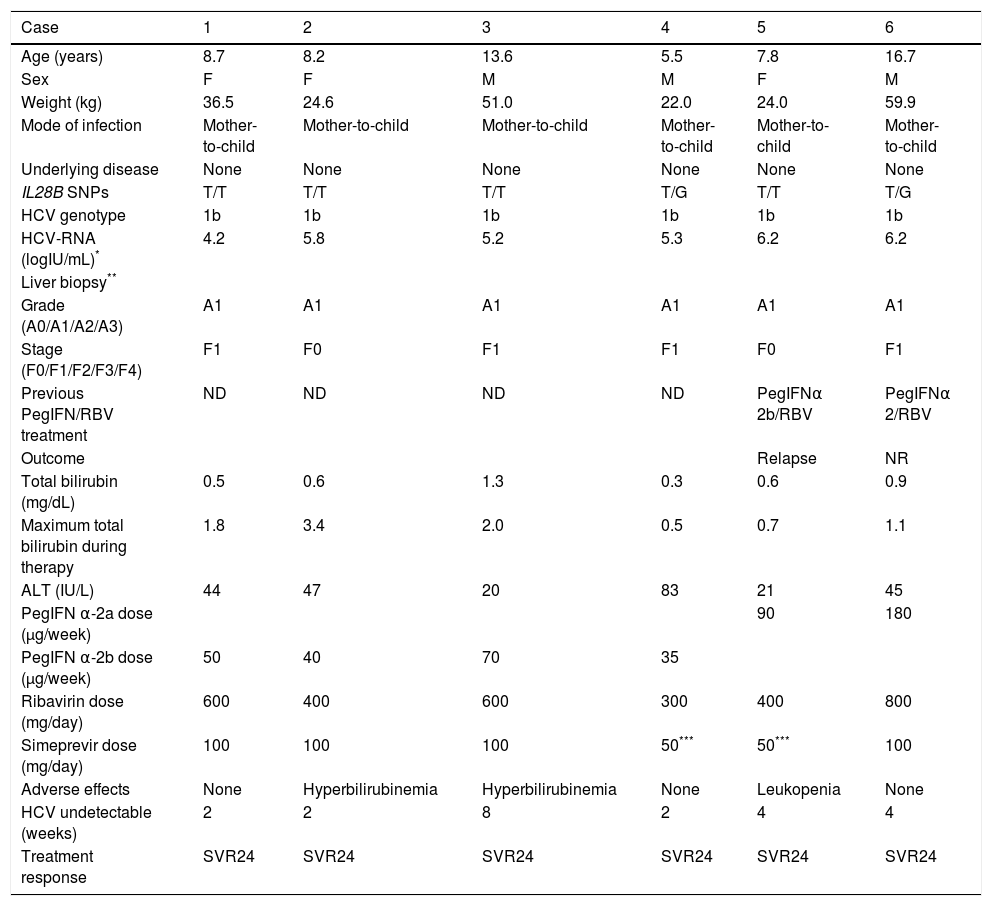
The Observatory for Hepatitis B and C Infection in Japanese Children was established in 2011 by the Hepatology Group of the Japanese Society for Pediatric Gastroenterology, Hepatology and Nutrition (JSPGHAN) with the aim of taking a census of children with hepatitis B and C infections and investigating the clinical aspects and outcomes of liver disease using an online registration system (http://kidhbvhcv.jp/index.php). This study was approved by all participating hospitals and their respective research ethics committees. Sixty-five pediatric centers in Japan were involved in this survey. Up to December 2016, six pediatric patients under 18 years of age received this triple therapy from pediatricians according to the JSPGHAN survey. The treatment schedule followed the criteria for response-guided therapy:2 5 patients (patients No. 1, 2, 4, 5, 6) received SMV for 12 weeks with peginterferon (PEG-IFN) α-2a/2b and ribavirin (RBV) followed by PEG-IFN/RBV for 12 weeks; and one patient (patient No. 3) received SMV for 24 weeks with PEG-IFNα-2b and RBV followed by PEG-IFN/RBV for 24 weeks. The rapid virological response (RVR) rate was 83% (5/6), and the SVR rate at 24 weeks post-treatment (SVR24) was 100% (Table 1). No severe adverse events were seen in any patients.
Characteristics of study patients receiving SMV/PEG-IFN/RBV combination therapy.
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (years) | 8.7 | 8.2 | 13.6 | 5.5 | 7.8 | 16.7 |
| Sex | F | F | M | M | F | M |
| Weight (kg) | 36.5 | 24.6 | 51.0 | 22.0 | 24.0 | 59.9 |
| Mode of infection | Mother-to-child | Mother-to-child | Mother-to-child | Mother-to-child | Mother-to-child | Mother-to-child |
| Underlying disease | None | None | None | None | None | None |
| IL28B SNPs | T/T | T/T | T/T | T/G | T/T | T/G |
| HCV genotype | 1b | 1b | 1b | 1b | 1b | 1b |
| HCV-RNA (logIU/mL)* | 4.2 | 5.8 | 5.2 | 5.3 | 6.2 | 6.2 |
| Liver biopsy** | ||||||
| Grade (A0/A1/A2/A3) | A1 | A1 | A1 | A1 | A1 | A1 |
| Stage (F0/F1/F2/F3/F4) | F1 | F0 | F1 | F1 | F0 | F1 |
| Previous PegIFN/RBV treatment | ND | ND | ND | ND | PegIFNα 2b/RBV | PegIFNα 2/RBV |
| Outcome | Relapse | NR | ||||
| Total bilirubin (mg/dL) | 0.5 | 0.6 | 1.3 | 0.3 | 0.6 | 0.9 |
| Maximum total bilirubin during therapy | 1.8 | 3.4 | 2.0 | 0.5 | 0.7 | 1.1 |
| ALT (IU/L) | 44 | 47 | 20 | 83 | 21 | 45 |
| PegIFN α-2a dose (μg/week) | 90 | 180 | ||||
| PegIFN α-2b dose (μg/week) | 50 | 40 | 70 | 35 | ||
| Ribavirin dose (mg/day) | 600 | 400 | 600 | 300 | 400 | 800 |
| Simeprevir dose (mg/day) | 100 | 100 | 100 | 50*** | 50*** | 100 |
| Adverse effects | None | Hyperbilirubinemia | Hyperbilirubinemia | None | Leukopenia | None |
| HCV undetectable (weeks) | 2 | 2 | 8 | 2 | 4 | 4 |
| Treatment response | SVR24 | SVR24 | SVR24 | SVR24 | SVR24 | SVR24 |
SMV: simeprevir. PEG-IFN: pegylated interferon. RBV: ribavirin. ND: not done. SNP: single nucleotide polymorphism. SVR: sustained virological response. NR: non responder. ALT: alanine aminotransferase. SVR24: sustained virological response at 24 weeks post-treatment. IL28B minor allele: heterozygotes (T/G) or homozygotes (G/G) of the minor allele (G). IL28B major allele: homozygotes for the major allele (T/T).
To compare the antiviral effects of SMV/PEG-IFN/ RBV therapy and PEG-IFN/RBV therapy, the decreases in the viral load were analyzed by calculating the changes in the viral load from day 0 (baseline) to days 14 and 28 of patients who achieved SVR24. Our published data of 8 patients (one patient had IL28B minor allele) who were infected with HCV genotype 1b and received PEG-IFN/ RBV therapy were used for the comparison.3 Differences in mean values between two groups were compared using the Mann-Whitney U-test using Prism software (v.7.0c; GraphPad Software, La Jolla, CA). The initial viral load was 6.43 ± 0.89 (mean ± SD) logIU/mL in the PEG-IFN/ RBV group and 5.74 ± 0.76 logIU/mL in the SMV/PEG-IFN/RBV group (p = 0.66). After 14 and 28 days of treatment, the decreases in viral load were 1.81 ± 0.58 logIU/ mL and 3.14 ± 0.87 logIU/mL, respectively, in the PEG-IFN/RBV group and 4.88 ± 0.68 logIU/mL and 5.28 ± 0.98 logIU/mL, respectively, in the SMV/PEG-IFN/RBV group (both p < 0.01).
Moderate neutropenia (<1.0 x 103/mm3) was observed in one patient (patient No. 5) who recovered without any changes to the treatment regimen. Transient and mild increases in total bilirubin levels were observed in 2 patients. Hyperbilirubinemia (total bilirubin > 2 mg/dL) was examined. In one patient (patient No. 3), total bilirubin values were elevated during the first 2 weeks, stabilized during continued treatment, and then returned to baseline values after week 12. The other patient (patient No. 2), whose total bilirubin value increased continuously and reached a maximum value of 3.4 mg/dL in week 6, discontinued oral administration of SMV in week 6. Bilirubin elevation began to decrease immediately after SMV was stopped, and it then returned to baseline after week 11. In these 2 patients, hyperbiliru-binemia was not associated with increases in either alanine aminotransferase or aspartate aminotransferase levels.
In PEG-IFN/RBV combination therapy, the treatment duration for patients with genotype 1 is 48 weeks. This combined therapy results in an SVR rate of up to 100% in HCV genotype 2 or 3 children, but of only 45% to 55% of those infected with genotype 1 or 4.4 In contrast, with SMV/PEG-IFN/RBV therapy with a shortened 24-week treatment duration, earlier viral clearance than that seen with PEG-IFN/RBV therapy was observed, and the SVR24 rate was 100% in the present survey, though the number of patients treated was small. This result provides further evidence supporting the added effectiveness that SMV offers as part of an IFN-based regimen. Since interferon-free DAA therapy, which provides a shorter, more effective, and better tolerated treatment,1 will eventually be approved for use in children younger than 12 years, further large-scale evidence about SMV/PEG-IFN/RBV will not be available in the future, except for the present series.
Long-term outcomes of 5-year follow-up of PEG-IFN/ RBV combination therapy were recently reported.5,6 No patients had relapsed in the 5-year follow-up period, but one report suggested that impairment of growth by PEG-IFN should be considered.6 Among patients treated for 24 weeks, full recovery of height z scores to baseline was observed by 1 year of follow-up, whereas only partial recovery was observed during 5 years of follow-up in patients treated for 48 weeks.6 The issue of growth inhibition could be resolved by introducing 24-week SMV/PEG-IFN/RBV therapy.
Hyperbilirubinemia is a commonly observed laboratory test abnormality during the initial 12 weeks of oral SMV administration, and it peaked 2 weeks after its administration was started. In vitro studies indicated that this may be due to the inhibition of organic anion-transporting polypeptides and multidrug resistance-associated protein 3 transporters by SMV, since both have a role in bilirubin clearance.7 Thus, a mild or moderate bilirubin increase during treatment is not usually considered to be due to deterioration caused by liver injury. Although close monitoring of side effects is needed, immediate drug discontinuation is not generally recommended for bilirubin increases. In the present study, oral SMV administration was discontinued because of hyperbilirubinemia in one patient who eventually achieved SVR. Consequently, SMV/PEG-IFN/RBV therapy was well tolerated in pediatric patients.
In conclusion, a regimen of IFN-based SMV could be used to improve the SVR rate in pediatric patients with HCV genotype 1 infection, without severe adverse effects and with shorter treatment time.
Abbreviations- •
DAAs: direct-acting antivirals.
- •
FDA: Food and Drug Administration.
- •
HCV: hepatitis C virus.
- •
IFN-based: interferon-based.
- •
JSPGHAN: Japanese Society for Pediatric Gastroen-terology, Hepatology and Nutrition.
- •
PEG-IFN: peginterferon.
- •
RBV: ribavirin.
- •
RVR: rapid virological response.
- •
SMV: simeprevir.
- •
SVR: sustained virological response.
Disclosure of potential conflicts of interest: All authors declare that they have no conflicts of interest.
FundingThis research was partially supported by grant 17fk0210108h0001 from the Japan Agency for Medical Research and Development (AMED).
AcknowledgementsThe authors would like to thank all participating patients and their families. The authors would also like to thank Satoshi Nakano (Department of Pediatrics, Juntendo University Faculty of Medicine), Tomoko Takano (Department of Pediatrics, Osaka General Medical Center) and Yoko Miyoshi (Department of Pediatrics, Osaka University Graduate School of Medicine) for collaborating with the data collection.