Research in the last few years has proven that inhibition of fatty acid synthase (FASN) suppresses the migration and invasion of hepatoma carcinoma cells. This study aims to explore the effect of fatty acid synthase knockdown on the apoptosis and proliferation of HepG2 cells.
Materials and methodsThe human liver cancer cell line HepG2 was cultured and then transfected with FASN-specific siRNA and negative control RNAi. After 48h, cells and protein lysates were used for western blotting, CCK-8 (cell counting kit-8) assays, flow cytometry and other tests. To assess cell apoptosis, Bax, Bcl-2 and caspase-3 were detected; to assess proliferation, CDK4 (cyclin-dependent kinases 4) and P21 were detected; and to determine the signaling pathway involved, β-catenin and C-myc were also detected.
ResultsInhibition of FASN in HepG2 cells can decrease proliferation and promote apoptosis. Flow cytometry and CCK-8 assays demonstrated that the apoptosis rate of FASN-specific siRNA-transfected cells was significantly increased compared to that of the control cells (p<0.01). In addition, the cell cycle analysis revealed that FASN-specific siRNA-transfected cells induced G1 phase arrest (p<0.05), but an increasing trend in G2 (p<0.05).
Compared with expression in negative RNAi-transfected cells, the expression of Bcl-2 and CDK-4 was reduced and the expression of Bax, caspase-3 and P21 was increased in FASN-specific siRNA-transfected cells (p<0.05). Regarding the signaling pathway, the expression of β-catenin and C-myc was significantly reduced when compared to that in negative control cells (p<0.05).
ConclusionsInhibition of FASN suppressed the cell survival of HepG2 cells by inhibiting the β-catenin/C-myc pathway. This result suggests the potential treatment value of FASN for hepatoma carcinoma (HCC).
With changes in lifestyle factors and socioeconomic factors, the burden of cancer has improved [1]. However, the high mortality rates and dire complications are still causes for concern. Of newly diagnosed cancer cases, liver cancer ranks sixth in incidence and fourth in mortality [1]. HCC is the most common form of liver cancer [2]. Viral hepatitis with advanced chronic liver disease contributed to the most of HCC cases, especially the hepatitis B virus (HBV) (accounts for at least 50% cases of HCC worldwide) and hepatitis C virus (HCV) infections are considered as major HCC risk factors [3–5]. Moreover, non-alcoholic steatohepatitis (NASH) is increasing as an etiology for end-stage liver disease as well as HCC, which because of its association with progressive fibrosis and cirrhosis [6]. Other causes of HCC also include exposure to aflatoxin, excessive smoking and metabolic syndromes. The epidemiological trends in HCC are changing due to various pathogenic and socioeconomic factors: HCC ranks the first in the fastest-rising cause of cancer-related deaths in America, North America and several European regions shows an increasing trend in the incidence and mortality of HCC, in contrast, a decreasing trend appeared in traditionally high-risk regions, including Japan and parts of China [7]. In addition to the differences of incidence of HCC of regions, there are also differences between genders. The incidence of HCC in men is 2–8 times higher than in women, which may because the sex hormones are closely related to the pathogenesis and development of HBV-induced HCC [3,8].
FASN regulates the synthesis of de novo fatty acids (FAs), which meets the need for cancer cells to compose more cell membranes [9–11]. According to numerous studies, the expression of FASN is closely related to cancer progression; in particular, a high expression level of FASN indicates poor prognosis, high recurrence rate and aggressiveness [12–15]. FASN has been proven to be associated with migration and invasion in HCC cells and osteosarcoma cells [16,17]. However, few studies have investigated its effects on apoptosis and proliferation.
Therefore, this study was conducted to explore the role of FASN in HepG2 cells. And the main methods included the detection of cell survival, cell proliferation, cell apoptosis, and β-catenin/C-myc signaling activity following FASN knockdown.
2Material and methods2.1Reagents and antibodiesCell lines. The human hepatocellular carcinoma cell line HepG2 was purchased from ATCC Cell Bank and incubated at 37°C in an atmosphere of 5% CO2 with 95% air. The cell culture medium was composed of high glucose DMEM (KeyGen Biotech, KGM12800-500, Jiangsu, China) plus 10% fetal bovine serum (FBS) (FSP100, Excell Bio, Shanghai, China).
2.2FASN siRNA transfectionTransfection can be done when cells accounts for 60–80% of container. In a six-well plate, the transfection dose for each well was 15μl of siRNA (HSS103565, Invitrogen, Carlsbad, CA, USA) or negative control RNAi (12935-400, Invitrogen, Carlsbad, CA, USA) at a concentration of 150nM in 250μl Opti-MEM (Gibco, San Diego, CA, USA) mixed with 5μl of Lipofectamine 2000 (Thermo Fishier Scientific, Carlsbad, CA, USA) in 250μl Opti-MEM incubated for 20min. The cells were cultured in high glucose DMEM plus 10% fetal bovine serum during transfection. In 24h, RNA can be extracted. In 48h, protein can be extracted for western blotting, CCK-8 and flow cytometry can be conducted.
2.3CCK-8 assayCell proliferation of FASN knockdown cells was detected by the CCK-8 assay (APExBIO, Houston, USA). To generate the survival curve, the transfected cells and negative control cells were diluted to ∼5×104cells/ml and incubated in a 96-well plate. Cells were fragile due to the cytotoxicity of Lipo2000, so we adjusted the detection interval to every 12h. Before every 12h of testing, 10μl CCK-8 per 100μl of cell suspension was added and cultured for 1h. Then, the optical density (OD) value was measured at 490nm by an ELX-800 microplate reader (Biotek Instruments, California, USA). The cell survival curve was generated from the OD value at each time point.
2.4Cell apoptosis and cell cycle analysisThe transfected cells were collected by centrifugation at 900rpm for 4min, then re-suspended in 2ml PBS. Divided the cell suspension into EP tubes and centrifuged at 3000rpm for 4min. For cell apoptosis evaluation, the cells were resuspended in PBS; for cell cycle analysis, the cells were resuspended in 75% alcohol; and sent to Institute of Life Science (Chongqing Medical University) for further flow cytometry detection.
2.5Western blottingForty-eight hours after transfection, cells were lysed with RIPA lysis buffer (Beyotime, Jiangsu, China), and the protein concentration was measured by a BCA protein detection kit. SDS-PAGE was performed and polyvinylidene difluoride (PVDF) membranes were used for electrophoresis and transfer, respectively. The transferred membranes were immersed in blocking solution (composed of skimmed milk powder and TBST) for 1.5h at room temperature. The antibodies to FASN (ab128870, 1:1000), β-catenin (AF6266, 1:1000), C-myc (TA500003S, 1:1000), P21 (AF6290, 1:1000), CDK4 (DF6102, 1:500) cleaved caspase-3 (AF7022, 1:1000), Bax (AF0120, 1:1000), and Bcl-2 (AF6139, 1:1000) were incubated with the membranes overnight at 4°C. Then, the membranes were washed with TBST and incubated in HRP-IgG antibodies (1:5000) for 1.5h at room temperature. The membranes were washed again before imaging. ECL reagent (Affinity Biosciences, Changzhou, China) was made at a ratio of 1:1, and bands were exposed via the measurement system (Bio-Rad Laboratories).
2.6Statistical analysisThe data from this study were presented as the mean±standard deviation (SD), and the experiments were conducted at least 3 times. GraphPad Prism (version 8.0) was applied for the data analysis and graph display. A p-value <0.05 indicates statistical significance between the experimental and control groups. “*” indicates a p-value <0.05, “**” indicates a p-value <0.01, “***” indicates a p-value <0.001 and “****” indicates a p-value <0.0001; these symbols are marked above the histograms.
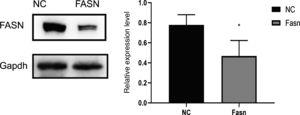
3ResultsKnockdown of FASN suppresses its expression in HepG2 cells. Protein was extracted after transfection for 48h. Then, western blotting was used to detect the expression of FASN at 270kDa. The expression level of the knockdown group was significantly lower than that of the negative control group, which indicates that FASN-specific siRNA can suppress the expression of FASN in HepG2 cells (Fig. 1).
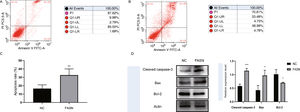
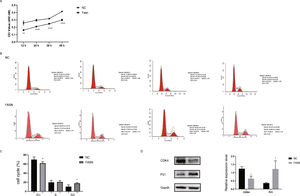
Knockdown of FASN inhibits proliferation and promotes apoptosis in HepG2 cells. Studies about the inhibition of FASN on the migration and invasion of HCC cells were reported previously; however, the effect on the apoptosis and proliferation of HepG2 cells is unknown [16,18,19]. In this study, apoptosis and proliferation were detected after FASN knockdown. The results showed an increasing rate of cell apoptosis (Fig. 2A–C). Moreover, the rate of apoptosis was closely related to the expression levels of cleaved caspase-3, Bax and Bcl-2 (Fig. 2D). The transfected cells were re-plated into 96-well plates for further CCK-8 detection, and the OD value at every 12h was tested for the cell survival curve (Fig. 3A). The cell cycle analysis revealed a significant G1 phase arrest in FASN knockdown cells with G2 phase increasing (Fig. 3B and C). The expression levels of CDK4 and P21 also indicated that the inhibition of FASN suppressed the proliferation of HepG2 cells (Fig. 3D).
(A) Representative images of the flow cytometry detection of negative control cells. (B) Representative images of the flow cytometry detection of FASN knockdown cells. (C) Quantitative analysis results of the flow cytometry of cell apoptosis in HepG2 cells. (D) Quantitative analysis results and representative images of the western blot results for cleaved caspase-3, Bax, and Bcl-2 in HepG2 cells.
(A) Quantitative analysis results of the CCK-8 assay of cell proliferation in HepG2 cells. (B) Images of flow cytometry detection of the cell cycle distribution in negative control and FASN knockdown cells. (C) Quantitative analysis results of flow cytometry detection of the cycle distribution. (D) Quantitative analysis results and representative images of the western blot results for CDK4 and P21 in HepG2 cells.
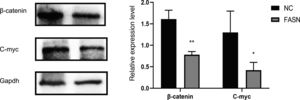
Knockdown of FASN suppresses the expression of the β-catenin/C-myc signaling pathway in HepG2 cells. This study was designed to investigate the downstream signaling pathway affected by FASN knockdown. Previous studies indicated that the transcription factor β-catenin enters the nucleus and regulates the pathway target gene C-myc in various kinds of cancers [20–23]. As shown in this study, the expression levels of β-catenin and C-myc in FASN knockdown cells were lower than those in negative control cells (Fig. 4).
4DiscussionThe five-year overall survival of HCC is less than 10%, which is not only due to the high mortality, invasiveness and resistance that make it difficult to cure, but is also due to the late diagnosis that contributes to the ineffectiveness of radiofrequency ablation (RFA) and liver transplantation treatment [24,25]. But in recent years, with technological innovation, the diagnosis of hepatic fibrosis in chronic hepatitis C virus has become more and more reliable, which has also promoted the prevention and diagnosis of HCC. For example, the apparent diffusion coefficient (ADC) value decreased significantly with the progression of fibrosis; plasma bile acid levels and the frequency of variant homozygote genotype CC increased significantly in advanced fibrosis; the value of cirrhosis risk score (CRS) ≥0.8 is predictive of patients with high risk for cirrhosis [26–28]. In addition to liver fibrosis and cirrhosis, which induces HCC, the rapid development of tumors is also associated with cell metabolism reprogramming, which is induced by oncogenic mutations [29–31]. FASN has a major role in regulating lipid metabolism, and many studies, through immunohistochemical staining, have proven that FASN is overexpressed in many malignant cancers, such as breast, prostate, colorectal, ovary, lung and liver cancers, but its expression is low in tumor-adjacent tissues [32–37]. The Wnt signaling pathway is a common pathway in cancer, and Guo et al. proved that the Wnt/β-catenin signaling pathway was involved in the apoptosis and proliferation of liver cancer cells [38]. β-Catenin, a key effector of the Wnt signaling pathway, accumulates in the nucleus and promotes the transcription of many oncogenes, such as C-myc, in many cancers [22,39]. According to the above studies, the goal of this study was to further investigate the effect of FASN knockdown on the β-catenin/C-myc pathway and the proliferation and apoptosis of HepG2 cells. The unique of this study is the conclusion that FSAN can suppress the apoptosis and proliferation of HepG2 cells in HCC. In addition, this study also tested the Wnt/β-catenin signaling pathway that has effects on cell apoptosis and proliferation, which was not implemented in other studies. Furthermore, compared with other studies using lentiviruses for transfection, this study focuses on siRNA transfection not only because of its higher transfection efficiency with smaller size, but also because of its convenient procedures, there are still some limitations. If the culture time of siRNA transfected cells is prolonged, the original siRNA mixture could not transfect the newly proliferated cells and the experimental results will be affected. Moreover, the next step must be performed immediately after transfected, due to the short utility time of siRNA transfection and cytotoxicity of Lipo2000.
Based on the results of this study, the apoptosis of FASN knockdown HepG2 cells demonstrated an increasing trend. In particular, the CCK-8 assay indicated a significant difference between the knockdown group and the negative control group: p<0.01 at 12h; p<0.0001 at 24h, 36h and 48h. Considering the cytotoxicity of Lipo2000, the time interval was set to 12h between every OD value detection. The G1 arrest of FASN knockdown cells in cell cycle evaluation also proved the inhibition of FASN can suppress the proliferation. However, G2 phase demonstrated an increasing trend in FASN knockdown cells, which probably due to the transfected cells proliferate into new cells, and this part of the cells has not been transfected. Moreover, flow cytometry also showed that the rate of apoptosis in FASN knockdown cells was greater than that in negative control cells (p<0.01) (Fig. 2C). Fig. 2A and B displays only representative images and quantitative analysis results of flow cytometry detection; the remaining flow cytometry detection data are included in supplementary material file 1. Additionally, the proliferation of FASN knockdown cells demonstrated a decreasing trend in parallel with the expression levels of CDK4 and P21. CDK4 is a key promoter of cell proliferation, and its proliferative effect in tumor cells occurs through silencing the CDK4 antagonist gene or enhancing CDK4 expression [40,41]. P21, as well as p21waf1/cip1 or P21/CDKN1A, is a CDK4 inhibitor with 165 amino acids [42,43]. In this study, the expression levels of CDK4 and P21 were inversely related in FASN knockdown cells; CDK4 expression was lower and P21 expression was higher in FASN knockdown cells than in negative control cells. These results showed that the inhibition of FASN suppressed proliferation and promoted apoptosis via the β-catenin/C-myc signaling pathway in HepG2 cells.
5ConclusionIn conclusion, the present study provides a potential treatment strategy for HCC by inhibiting FASN to suppress the proliferation and promote the apoptosis of HCC cells via the β-catenin/C-myc signaling pathway in vitro. Further study is required to determine the effects on other HCC cells.AbbreviationsFASN fatty acid synthase cell counting kit-8 cyclin-dependent kinases 4 hepatoma carcinoma hepatitis B virus hepatitis C virus non-alcoholic steatohepatitis fetal bovine serum optical density polyvinylidene difluoride radiofrequency ablation apparent diffusion coefficient cirrhosis risk score
Wenyue Zhang and Juan Huang contributed to the conduction and design of the study. Wenyue Zhang, Juan Huang, Yao Tang and Yixuan Yang performed the statistical analyses and drafted the manuscript. Huaidong Hu supervised the study. All authors gave final approval.
FundingThis study was supported by the Natural Science Foundation of China (grant no. 81171560), the “Par-Eu Scholars Program” of Chongqing City and the National Science and Technology Major Project of China (grant no. 2012ZX10002007001). The funding bodies had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflicts of interestsNone of authors have any financial discloser or conflict of interest.