Multidrug-resistant (MDR) infections in cirrhosis are associated with poor outcomes. We attempted a prospective study on infections in patients with cirrhosis evaluating microbiology of these infections and how outcomes depended on factors like bacterial resistance, appropriate antibiotics, stage of liver disease and whether outcomes were significantly different from patients who did not have infections.
Materials and methodsThis was a prospective evaluation involving one hundred and fifty nine patients with cirrhosis who were admitted at Peerless Hospitex Hospital and Research Center, Kolkata, West Bengal, India, during a 24 month period. One hundred and nineteen of these patients either had an infection at the time of admission or developed infection during hospitalization. Forty patients did not have an infection at admission and did not acquire infection while admitted. Data was collected about demographics, etiology of cirrhosis, liver and renal function and microbiology.
ResultsInfections were community acquired in 27.7% of patients, healthcare associated in 52.9% and nosocomial in 19.3%. Gram negative bacilli (Escherichia coli 47.4% Klebsiella pneumoniae 23%) were common. 84.9% of enterobacteriaceae produced ESBL, AmpC or Carbapenemases. Spontaneous bacteria peritonitis (SBP) and urinary tract infection (UTI) were the most common sites of infection. In hospital mortality was 21.9%. Non-survivors had higher MELD (26 vs 19, p<0.001) and CTP scores (11.7 vs 10.3, p<0.001). The control group had lower MELD (16.65 vs. 20.8, p<0.001) and CTP scores (9.25 vs 10.59, p<0.001).
ConclusionsMDR infections are common in patients with cirrhosis and have serious implications for treatment and outcomes.
Infection is one of the commonest events leading to hospital admissions in cirrhotic patients. These patients are prone to infections because of immune dysfunction, bacterial translocation and the altered gut microbiome in cirrhosis. In fact bacterial infections can act as a triggering factor for development of hepatic encephalopathy, gastrointestinal bleeding, acute kidney injury and acute on chronic liver failure. Therefore they represent an important cause of increased mortality [1].
In 2014 a position paper was published from the European Association for the Study of the Liver (EASL) highlighting these issues [1]. It has been mentioned that most of these patients suffer from infections caused by Gram negative enterobacteriaciae though Gram positive bacterial infections are not uncommon in hospitalized patients. Multidrug-resistant (MDR) bacterial infections are becoming common across the continents as reported from Korea [2] {29% ESBL (Extended Spectrum Beta Lactamase) producing enterobacteriaciae}, Italy [3] {8% ESBL producing enterobacteriaciae}, Spain [4] {6% ESBL, 2% Pseudomonas, 2% Acinetobacter, 1% VSE (Vancomycin Sensitive Enterococci)}, France [5] {8% MRSA (Methicillin Resistant Staphylococcus aureus), 5% VSE, 4% ESBL}, Germany [6] {10% VSE} and USA [7] {9% VRE (Vancomycin Resistant Enterococci), 6.5% ESBL, 5% MRSA}. It has been shown that MDR infections are responsible for higher initial treatment failure, higher hospital mortality and in some studies are identified as independent predictors of mortality at 30 days [8,9].
About one third of patients hospitalized with cirrhosis have at least one infection and two thirds of such infections are healthcare associated or nosocomial in origin [9]. Therefore first line antibiotics are often ineffective and may be responsible for high failure rate and mortality [10]. There is a paucity of Indian data on microbiology of infection in cirrhosis and their impact on outcomes of admitted patients with cirrhosis. We undertook the present study, prospectively following a cohort of cirrhotic patients during hospitalization. We analyzed microbiological data with special reference to patterns of antibiotic resistance and whether in hospital patient outcomes were influenced by drug resistance and appropriate antibiotic therapy. A control group was also recruited which represented patients who did not have infection during their hospital stay so that we could establish whether presence of infection itself caused worse outcomes. Our aim was to attempt a prospective study looking at infections in patients with cirrhosis and to document the microbiology of these infections with special reference to bacterial drug resistance. We looked at how outcomes depended on factors such as bacterial resistance, appropriate antibiotic therapy and stage of liver disease. We also attempted to analyze whether outcomes were significantly different from patients who did not have infections.
2Materials and methodsThe study is designed as a prospective observational study. All patients diagnosed with cirrhosis of liver and admitted either with an infection or who developed an infection during the course of hospital stay were included in the study. All patients who satisfied inclusion criteria and were admitted to hospital over a 24 month period from June 2013 to June 2015 were included. We also recruited patients with cirrhosis admitted during the same period who did not have infection at the time of admission and who also did not acquire an infection during the period of their stay in hospital The study protocol was approved by our hospital ethics committee (Ref: PH&BKRRCCREC/1093/2013). Written informed consent was obtained from all patients or the next of kin prior to enrolment.
Exclusion criteria were human immunodeficiency virus infection, previous transplantation and any other type of immunodeficiency. Diagnosis of cirrhosis was established by histology or by clinical, analytical and ultrasonographic findings [9]. Data was collected regarding age, sex, etiology of cirrhosis, presence of co-morbid conditions.
Infections were defined as follows:
- 1.
Spontaneous bacteremia: positive blood cultures without a source of infection [9,11].
- 2.
Spontaneous Bacterial Peritonitis: Ascitic fluid polymorphonuclear cells>250/μL with or without a positive fluid culture [9,12–14].
- 3.
Lower Respiratory Tract Infection: New pulmonary infiltrate in the presence of a) at least one respiratory symptom (cough, sputum production, dyspnea or pleuritic pain)+b) at least one finding on auscultation (rhonchi or creps) or one sign of infection (core body temperature>38C or <36C or TLC (Total Leukocyte Count)>10,000 or <4000/cumm [8,15].
- 4.
Bacterial enterocolitis: Diarrhea or dysentery with a positive stool culture for Salmonella, Shigella, Campylobacter, Yersinia or pathogenic Escherichia coli[8,15].
- 5.
Skin infection: Fever with cellulitis [8,24].
- 6.
Urinary Tract Infection: Symptoms consistent with cystitis or pyelonephritis along with>10pus cells/hpf on urine microscopy with significant bacteriuria [8,15].
- 7.
Diagnosis of other infections was made according to conventional criteria [16].
Data was also collected in relation to mode of acquisition of infection. Mode of acquisition was defined as [8,17,18]
- 1.
Community Acquired (CA): Diagnosed within 48h of admission without hospitalization in the previous 6 months.
- 2.
Health Care Associated (HCA): Infections diagnosed at admission or within 48h of admission in those patients with previous contact with a healthcare environment (e.g. hospitalization or short term admission for at least 2 days in the previous 180 days, residence in a nursing home or a long term care facility or chronic hemodialysis).
- 3.
Nosocomial (NC): Diagnosed beyond 48h of admission.
All the samples were collected and processed as per standard protocol. Blood cultures were done by BACTEC 9050 Becton Dickinson 22 Sawgrass Drive Bellport, NY 11713 USA. All positive growths were further evaluated. Identification and antimicrobial susceptibility testing were done by fully automated VITEK-2 Compact system bioMerieux Marcy-l’Etoile, France. Clinical and Laboratory Standards Institute (CLSI) breakpoints were considered while determining antimicrobial susceptibility. Bacterial resistance mechanisms and phenotypes were detected by the Advanced Expert System (AES), which is a critical component of VITEK-2 technology.
The following bacteria were considered to be MDR in the present study: ESBL, AmpC or carbapenemase producing enterobacteriaceae described as ESBL E, Pseudomonas aeroginosa, Acinetobacter baumanii, MRSA and Enterococcus faecium.
The clinical course was assessed by efficacy of empirical antibiotic therapy, complications related to infection, resolution and in hospital mortality. Empirical antibiotics used in our study were as follows: (1) Piperacillin tazobactam, Cefoperazone sulbactam or Cefepime for Community Acquired (CA) or Healthcare Associated (HCA) spontaneous bacterial peritonitis, spontaneous bacteremia, UTI and sepsis of unknown origin who were hemodynamically stable and not in shock. Ertapenem was also used for UTI. (2) Group 2 Carbapenems such as Meropenem or Imipenem along with Teicoplanin and Colistin (if considered appropriate) for Nosocomial (NC) infections or patients with shock (3) Piperacillin tazobactam plus Levofloxacin for Pneumonia (3) Group 2 Carbapenem plus Teicoplanin (and colistin) for ventilator associated pneumonia.
Criteria used to consider an infection cured were the following [9]: (1) no clinical signs of infection (2) <250polymorphonuclear cells/cumm in ascitic fluid for SBP (Spontaneous Bacterial Peritonitis) (3) Negative control cultures after antibiotic treatment for UTI. Resolution of other infections was established by conventional criteria [17].
2.1Statistical methodsCategorical variables are expressed as Number of patients and percentage of patients and compared across the groups using Pearson's Chi Square test for Independence of Attributes.
Continuous variables are expressed as Mean and Standard Deviation and compared across the 2 groups using Mann–Whitney U test.
The statistical software SPSS version 20 has been used for the analysis.
An alpha level of 5% has been taken, i.e. if any p value is less than 0.05 it has been considered as significant.
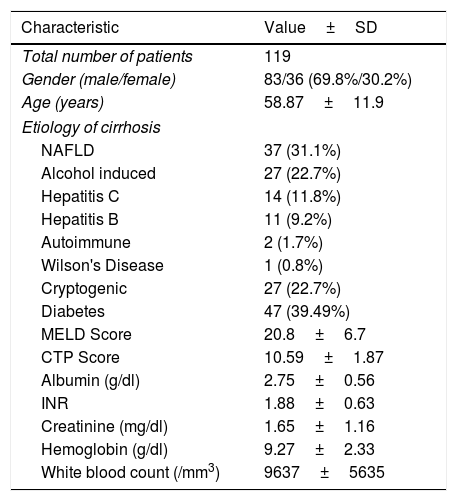
3ResultsWe identified a total of 119 patients who suffered infection during the study period. Demographic details and data related to cirrhosis etiology are presented in Table 1. There was a male preponderance in the cohort (69.8%). In the cohort Non alcoholic fatty liver disease (NAFLD) (31.1%) and ethanol induced cirrhosis (22.7%) represented the commonest etiology of cirrhosis. 27 patients (22.7%) in whom etiology could not be established were considered to have cryptogenic cirrhosis. Among significant co-morbidities 47 patients (39.49%) had Diabetes mellitus. The mean Model for End Stage Liver Disease (MELD) score on admission was 20.8 and Child-Turcotte-Pugh (CTP) score 10.59. Other baseline laboratory data are presented in Table 1.
Demographic Data and Baseline Lab Values in 119 patients with cirrhosis.
| Characteristic | Value±SD |
|---|---|
| Total number of patients | 119 |
| Gender (male/female) | 83/36 (69.8%/30.2%) |
| Age (years) | 58.87±11.9 |
| Etiology of cirrhosis | |
| NAFLD | 37 (31.1%) |
| Alcohol induced | 27 (22.7%) |
| Hepatitis C | 14 (11.8%) |
| Hepatitis B | 11 (9.2%) |
| Autoimmune | 2 (1.7%) |
| Wilson's Disease | 1 (0.8%) |
| Cryptogenic | 27 (22.7%) |
| Diabetes | 47 (39.49%) |
| MELD Score | 20.8±6.7 |
| CTP Score | 10.59±1.87 |
| Albumin (g/dl) | 2.75±0.56 |
| INR | 1.88±0.63 |
| Creatinine (mg/dl) | 1.65±1.16 |
| Hemoglobin (g/dl) | 9.27±2.33 |
| White blood count (/mm3) | 9637±5635 |
CTP: Child Turcott Pugh; INR: International Normalized Ratio; MELD: Model for End Stage Liver Disease; NAFLD: Non Alcoholic Fatty Liver Disease.
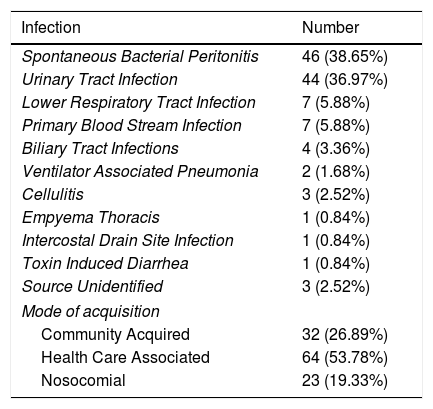
Site of infection is represented in Table 2. SBP 46 (38.65%) and UTI 44 (36.97%) represented the vast majority of infections. 33 infections (27.73%) were community acquired, 63 (52.94%) were health care associated and 23 (19.33%) were nosocomial.
Site of infection and mode of acquisition.
| Infection | Number |
|---|---|
| Spontaneous Bacterial Peritonitis | 46 (38.65%) |
| Urinary Tract Infection | 44 (36.97%) |
| Lower Respiratory Tract Infection | 7 (5.88%) |
| Primary Blood Stream Infection | 7 (5.88%) |
| Biliary Tract Infections | 4 (3.36%) |
| Ventilator Associated Pneumonia | 2 (1.68%) |
| Cellulitis | 3 (2.52%) |
| Empyema Thoracis | 1 (0.84%) |
| Intercostal Drain Site Infection | 1 (0.84%) |
| Toxin Induced Diarrhea | 1 (0.84%) |
| Source Unidentified | 3 (2.52%) |
| Mode of acquisition | |
| Community Acquired | 32 (26.89%) |
| Health Care Associated | 64 (53.78%) |
| Nosocomial | 23 (19.33%) |
Community Acquired (CA): Diagnosed within 48h of admission without hospitalization in the previous 6 months; Health Care Associated (HCA): Infections diagnosed at admission or within 48h of admission in those patients with previous contact with a healthcare environment (e.g. hospitalization or short term admission for at least 2 days in the previous 180 days, residence in a nursing home or a long term care facility or chronic hemodialysis); Nosocomial (NC): Diagnosed beyond 48h of admission.
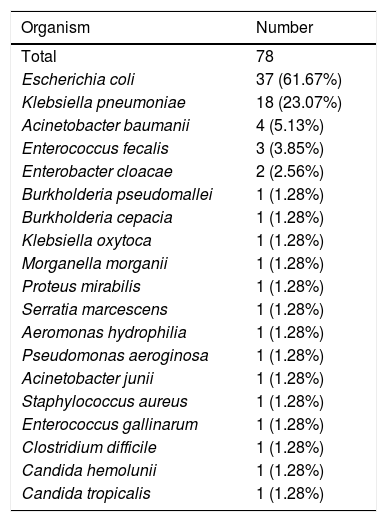
The total number of pathogens isolated was 78 (Table 3). 97.4% of isolated organisms were bacteria with 89.7% being gram negative bacteria. E. coli and Klebsiella pneumoniae accounted for 70.5% of the isolated organisms.
Microbiological isolates obtained from 78 patients.
| Organism | Number |
|---|---|
| Total | 78 |
| Escherichia coli | 37 (61.67%) |
| Klebsiella pneumoniae | 18 (23.07%) |
| Acinetobacter baumanii | 4 (5.13%) |
| Enterococcus fecalis | 3 (3.85%) |
| Enterobacter cloacae | 2 (2.56%) |
| Burkholderia pseudomallei | 1 (1.28%) |
| Burkholderia cepacia | 1 (1.28%) |
| Klebsiella oxytoca | 1 (1.28%) |
| Morganella morganii | 1 (1.28%) |
| Proteus mirabilis | 1 (1.28%) |
| Serratia marcescens | 1 (1.28%) |
| Aeromonas hydrophilia | 1 (1.28%) |
| Pseudomonas aeroginosa | 1 (1.28%) |
| Acinetobacter junii | 1 (1.28%) |
| Staphylococcus aureus | 1 (1.28%) |
| Enterococcus gallinarum | 1 (1.28%) |
| Clostridium difficile | 1 (1.28%) |
| Candida hemolunii | 1 (1.28%) |
| Candida tropicalis | 1 (1.28%) |
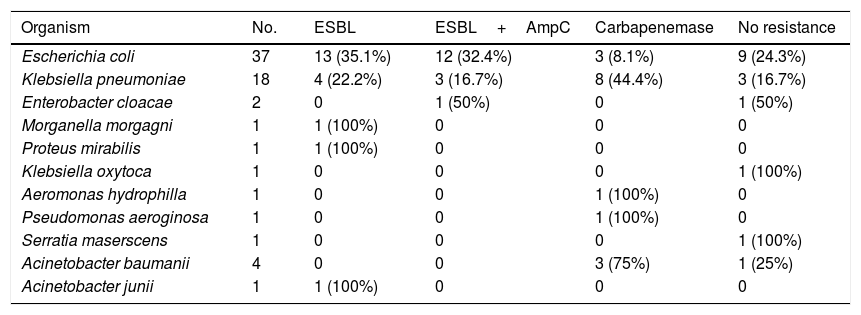
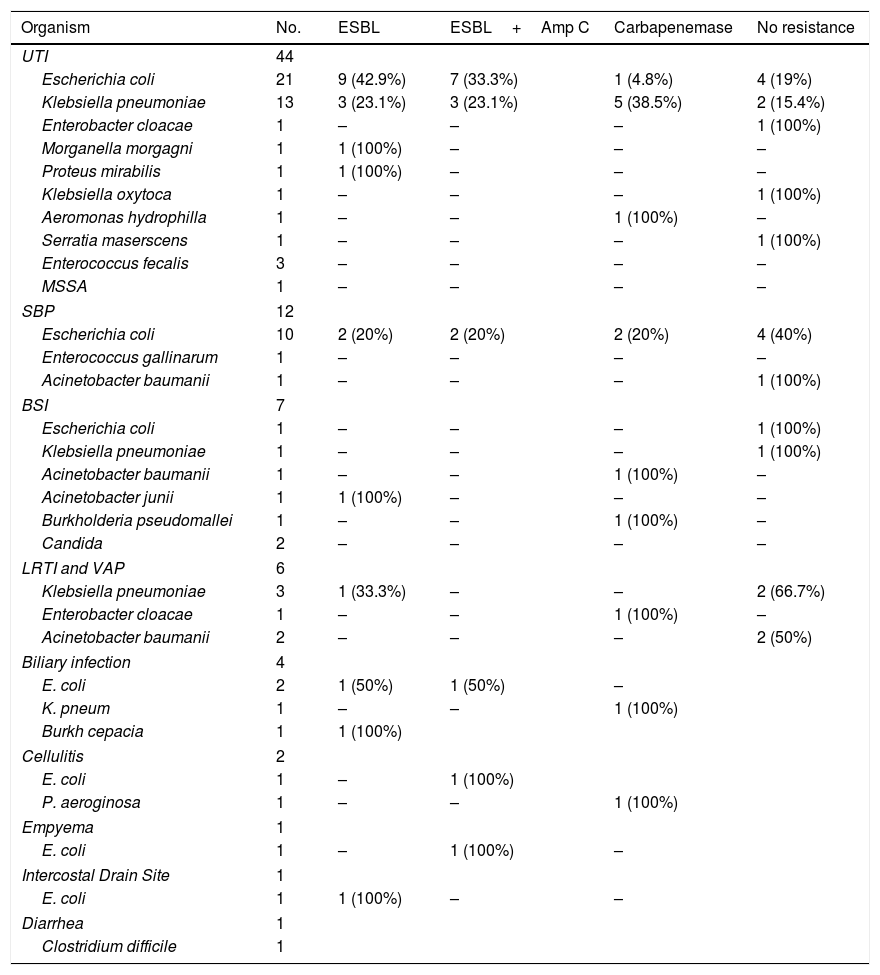
We analyzed resistance patterns in Enterobacteriaceae and other Gram negative bacteria (Table 4). We had high rates of drug resistant pathogens with 84.9% of Enterobacteriaceae producing ESBL, ESBL and AmpC or Carbapenemases. Among the A. baumanii 3 isolates (75%) were carbapenemase producers as was the only isolate of P. aeroginosa. Table 5 represents distribution of bacterial isolates according to site of infection and also patterns of resistance.
Production of ESBL, ESBL and AmpC and Carbapenemases in isolated gram negative bacteria.
| Organism | No. | ESBL | ESBL+AmpC | Carbapenemase | No resistance |
|---|---|---|---|---|---|
| Escherichia coli | 37 | 13 (35.1%) | 12 (32.4%) | 3 (8.1%) | 9 (24.3%) |
| Klebsiella pneumoniae | 18 | 4 (22.2%) | 3 (16.7%) | 8 (44.4%) | 3 (16.7%) |
| Enterobacter cloacae | 2 | 0 | 1 (50%) | 0 | 1 (50%) |
| Morganella morgagni | 1 | 1 (100%) | 0 | 0 | 0 |
| Proteus mirabilis | 1 | 1 (100%) | 0 | 0 | 0 |
| Klebsiella oxytoca | 1 | 0 | 0 | 0 | 1 (100%) |
| Aeromonas hydrophilla | 1 | 0 | 0 | 1 (100%) | 0 |
| Pseudomonas aeroginosa | 1 | 0 | 0 | 1 (100%) | 0 |
| Serratia maserscens | 1 | 0 | 0 | 0 | 1 (100%) |
| Acinetobacter baumanii | 4 | 0 | 0 | 3 (75%) | 1 (25%) |
| Acinetobacter junii | 1 | 1 (100%) | 0 | 0 | 0 |
ESBL: Extended Spectrum Beta Lactamases.
Microbial isolates according to site of infection with patterns of resistance.
| Organism | No. | ESBL | ESBL+Amp C | Carbapenemase | No resistance |
|---|---|---|---|---|---|
| UTI | 44 | ||||
| Escherichia coli | 21 | 9 (42.9%) | 7 (33.3%) | 1 (4.8%) | 4 (19%) |
| Klebsiella pneumoniae | 13 | 3 (23.1%) | 3 (23.1%) | 5 (38.5%) | 2 (15.4%) |
| Enterobacter cloacae | 1 | – | – | – | 1 (100%) |
| Morganella morgagni | 1 | 1 (100%) | – | – | – |
| Proteus mirabilis | 1 | 1 (100%) | – | – | – |
| Klebsiella oxytoca | 1 | – | – | – | 1 (100%) |
| Aeromonas hydrophilla | 1 | – | – | 1 (100%) | – |
| Serratia maserscens | 1 | – | – | – | 1 (100%) |
| Enterococcus fecalis | 3 | – | – | – | – |
| MSSA | 1 | – | – | – | – |
| SBP | 12 | ||||
| Escherichia coli | 10 | 2 (20%) | 2 (20%) | 2 (20%) | 4 (40%) |
| Enterococcus gallinarum | 1 | – | – | – | – |
| Acinetobacter baumanii | 1 | – | – | – | 1 (100%) |
| BSI | 7 | ||||
| Escherichia coli | 1 | – | – | – | 1 (100%) |
| Klebsiella pneumoniae | 1 | – | – | – | 1 (100%) |
| Acinetobacter baumanii | 1 | – | – | 1 (100%) | – |
| Acinetobacter junii | 1 | 1 (100%) | – | – | – |
| Burkholderia pseudomallei | 1 | – | – | 1 (100%) | – |
| Candida | 2 | – | – | – | – |
| LRTI and VAP | 6 | ||||
| Klebsiella pneumoniae | 3 | 1 (33.3%) | – | – | 2 (66.7%) |
| Enterobacter cloacae | 1 | – | – | 1 (100%) | – |
| Acinetobacter baumanii | 2 | – | – | – | 2 (50%) |
| Biliary infection | 4 | ||||
| E. coli | 2 | 1 (50%) | 1 (50%) | – | |
| K. pneum | 1 | – | – | 1 (100%) | |
| Burkh cepacia | 1 | 1 (100%) | |||
| Cellulitis | 2 | ||||
| E. coli | 1 | – | 1 (100%) | ||
| P. aeroginosa | 1 | – | – | 1 (100%) | |
| Empyema | 1 | ||||
| E. coli | 1 | – | 1 (100%) | – | |
| Intercostal Drain Site | 1 | ||||
| E. coli | 1 | 1 (100%) | – | – | |
| Diarrhea | 1 | ||||
| Clostridium difficile | 1 | ||||
BSI: Bloodstream infection; ESBL: Extended Spectrum Beta Lactamases; LRTI: Lower respiratory tract infection; SBP: Spontaneous Bacterial Peritonitis; UTI: Urinary tract infection; VAP: Ventilator associated pneumonia.
Resistance to other antibiotics was also observed with 11.5% being resistant to aminoglycosides, 51.7% resistant to Trimethoprim sulphamethoxazole and 73.8% resistant to Fluoroquinolones.
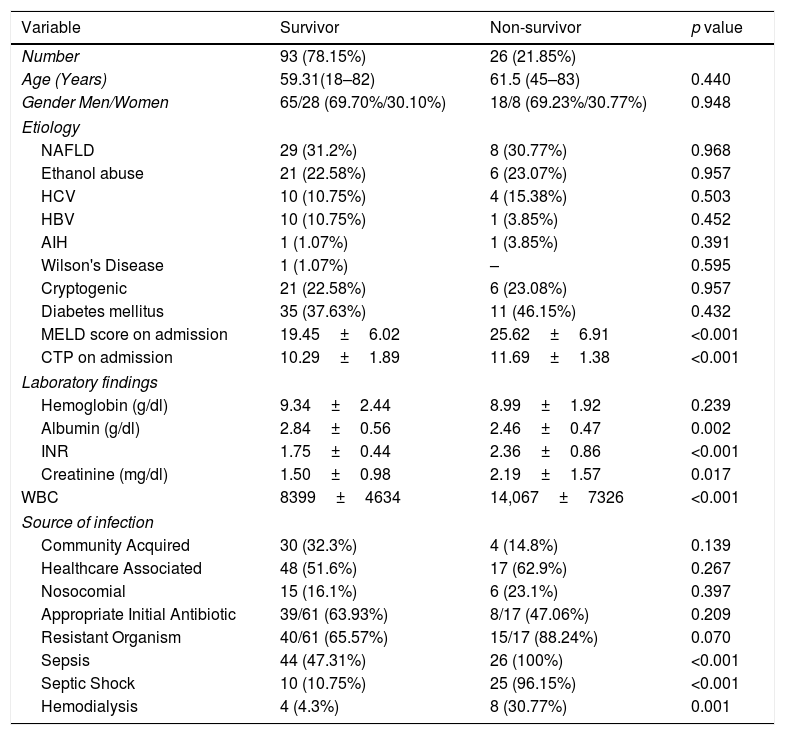
Adverse outcomes in our patients were documented. Twenty six patients died during the index hospitalization (21.85%). 12 patients (10.08%) required renal replacement therapy. We also compared hospital survivors and non-survivors. Comparison was done with respect to etiology of cirrhosis, presence of Diabetes mellitus, MELD score on admission, baseline hemoglobin, albumin, INR, creatinine and white blood count, source of acquisition of infection, whether they had sepsis or septic shock and whether appropriate antibiotics were administered initially as per subsequent culture reports in culture positive patients (Table 6).
Comparison of patient characteristics between hospital survivors and non-survivors.
| Variable | Survivor | Non-survivor | p value |
|---|---|---|---|
| Number | 93 (78.15%) | 26 (21.85%) | |
| Age (Years) | 59.31(18–82) | 61.5 (45–83) | 0.440 |
| Gender Men/Women | 65/28 (69.70%/30.10%) | 18/8 (69.23%/30.77%) | 0.948 |
| Etiology | |||
| NAFLD | 29 (31.2%) | 8 (30.77%) | 0.968 |
| Ethanol abuse | 21 (22.58%) | 6 (23.07%) | 0.957 |
| HCV | 10 (10.75%) | 4 (15.38%) | 0.503 |
| HBV | 10 (10.75%) | 1 (3.85%) | 0.452 |
| AIH | 1 (1.07%) | 1 (3.85%) | 0.391 |
| Wilson's Disease | 1 (1.07%) | – | 0.595 |
| Cryptogenic | 21 (22.58%) | 6 (23.08%) | 0.957 |
| Diabetes mellitus | 35 (37.63%) | 11 (46.15%) | 0.432 |
| MELD score on admission | 19.45±6.02 | 25.62±6.91 | <0.001 |
| CTP on admission | 10.29±1.89 | 11.69±1.38 | <0.001 |
| Laboratory findings | |||
| Hemoglobin (g/dl) | 9.34±2.44 | 8.99±1.92 | 0.239 |
| Albumin (g/dl) | 2.84±0.56 | 2.46±0.47 | 0.002 |
| INR | 1.75±0.44 | 2.36±0.86 | <0.001 |
| Creatinine (mg/dl) | 1.50±0.98 | 2.19±1.57 | 0.017 |
| WBC | 8399±4634 | 14,067±7326 | <0.001 |
| Source of infection | |||
| Community Acquired | 30 (32.3%) | 4 (14.8%) | 0.139 |
| Healthcare Associated | 48 (51.6%) | 17 (62.9%) | 0.267 |
| Nosocomial | 15 (16.1%) | 6 (23.1%) | 0.397 |
| Appropriate Initial Antibiotic | 39/61 (63.93%) | 8/17 (47.06%) | 0.209 |
| Resistant Organism | 40/61 (65.57%) | 15/17 (88.24%) | 0.070 |
| Sepsis | 44 (47.31%) | 26 (100%) | <0.001 |
| Septic Shock | 10 (10.75%) | 25 (96.15%) | <0.001 |
| Hemodialysis | 4 (4.3%) | 8 (30.77%) | 0.001 |
AIH: Autoimmune Hepatitis, CTP: Child Turcotte-Pugh, HBV: Hepatitis B Virus, HCV: Hepatitis C Virus, INR: International Normalized Ratio, MELD: Model for End Stage Liver Disease, NAFLD: Non Alcoholic Fatty Liver Disease, WBC: White Blood Count.
With regards to etiology of cirrhosis there was no significant difference between the 2 groups. 46.15% of non-survivors had Diabetes mellitus compared to 37.63% of survivors. When we compared MELD and laboratory parameters a statistically significant difference was demonstrated with regards to MELD score (p<0.001), Child–Pugh score (p<0.001), INR (p<0.001and white blood count (p<0.001). There was no statistically significant difference between the 2 groups in terms of mode of acquisition of infection. There were statistically significant differences in terms of how many patients had sepsis (p<0.001) and septic shock (p<0.001). There was no statistically significant difference between the 2 groups in terms of number of patients who were infected with a resistant organism and received an appropriate antibiotic initially.
Role of empirical antibiotics: 66 (55.46%) patients received a Beta lactam/beta lactamase (Piperacillin Tazobactam, Cefoperazone Sulbactam, Ticarcillin Clavulanic Acid or Cefepime Tazobactam) as the initial antibiotic choice. 13 patients (10.92%) received Cefepime, 5 patients (4.20%) received Ertapenem, 29 patients (24.37%) received a group 2 carbapenem (Imipenem or Meropenem) as the empiric choice,12 patients (10.08%) received MRSA cover as part of the initial regime in the form of Clindamycin, Linezolid or Teicoplanin. 1 patient received colistin monotherapy as the initial antibiotic and 2 patients with nosocomial infections received Meropenem/Imipenem with Teicoplanin, Colistin and FCZ as initial therapy.
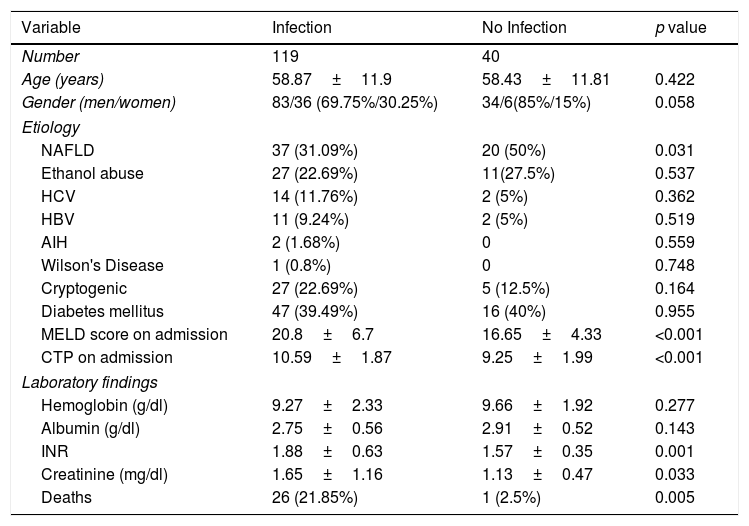
During the study period we also enrolled 40 patients with cirrhosis who did not have infection at the time of admission and were not diagnosed with any infections during the admission. There demographic data, laboratory values and etiology of cirrhosis are presented in Table 7.
Comparison of characteristics between patients with and without infection.
| Variable | Infection | No Infection | p value |
|---|---|---|---|
| Number | 119 | 40 | |
| Age (years) | 58.87±11.9 | 58.43±11.81 | 0.422 |
| Gender (men/women) | 83/36 (69.75%/30.25%) | 34/6(85%/15%) | 0.058 |
| Etiology | |||
| NAFLD | 37 (31.09%) | 20 (50%) | 0.031 |
| Ethanol abuse | 27 (22.69%) | 11(27.5%) | 0.537 |
| HCV | 14 (11.76%) | 2 (5%) | 0.362 |
| HBV | 11 (9.24%) | 2 (5%) | 0.519 |
| AIH | 2 (1.68%) | 0 | 0.559 |
| Wilson's Disease | 1 (0.8%) | 0 | 0.748 |
| Cryptogenic | 27 (22.69%) | 5 (12.5%) | 0.164 |
| Diabetes mellitus | 47 (39.49%) | 16 (40%) | 0.955 |
| MELD score on admission | 20.8±6.7 | 16.65±4.33 | <0.001 |
| CTP on admission | 10.59±1.87 | 9.25±1.99 | <0.001 |
| Laboratory findings | |||
| Hemoglobin (g/dl) | 9.27±2.33 | 9.66±1.92 | 0.277 |
| Albumin (g/dl) | 2.75±0.56 | 2.91±0.52 | 0.143 |
| INR | 1.88±0.63 | 1.57±0.35 | 0.001 |
| Creatinine (mg/dl) | 1.65±1.16 | 1.13±0.47 | 0.033 |
| Deaths | 26 (21.85%) | 1 (2.5%) | 0.005 |
AIH: Autoimmune Hepatitis, CTP: Child Turcotte-Pugh, HBV: Hepatitis B Virus, HCV: Hepatitis C Virus, INR: International Normalized Ratio, MELD: Model for End Stage Liver Disease, NAFLD: Non Alcoholic Fatty Liver Disease.
We also compared the baseline characteristics of the group of patients with infection with those without infection. Statistically significant differences were seen with regards to MELD, Child's score, INR and creatinine (Table 7).
4DiscussionInfections account for a significant degree of morbidity and mortality in patients with cirrhosis and will account for a significant proportion of deaths. Patients with cirrhosis are more prone to develop infections due to a number of factors including decreased immunity and increased bacterial translocation from the gut. In our study conducted in a tertiary hospital in Eastern India we looked at patients with cirrhosis who were either admitted with infectious complications or developed infections during their hospital stay. We looked at the epidemiology of the infections and patterns of antibiotic resistance and empirical antibiotic use. Correlation between outcome of index hospitalization and degree of liver impairment as measured by MELD and Child–Pugh scores was also analyzed.
The mean age in our study population was 58.87 years which is slightly older than in most other similar studies [19–21]. The mean MELD score was 20.8. The average CTP score was 10.59 placing a majority of our patients in Child Class C. This indicates that our population comprised a larger number of older individuals with more advanced liver disease. The in hospital mortality rate in our study was 21.85% which is comparable to most other studies which vary from 14 to 30% [19–21]. Mortality in our study was seen to be associated with higher MELD scores, higher CTP scores, lower albumin, higher INR and higher baseline creatinine and total leukocyte counts and these associations were seen to be statistically significant. There was also an association between mortality and lower hemoglobin. These findings have been reciprocated in most similar studies and would be expected as all of these characterize more advanced liver disease.
Our series showed that the two commonest infections were SBP (38.65%) and UTI (36.97%). This is roughly what has been demonstrated in other studies [19–21].
The prevalence of drug resistant pathogens was high in our group of patients. Among the 61 positive cultures for enterobacteriaceae only 15 organisms (24.59%) showed neither ESBL, AmpC nor Carbapenemase production. Among our Enterobacteriaciae isolates 31.1% were ESBL producers, 26.2% were ESBL and AmpC producers and 19.7% were carbapenemase producers. Incidence of resistance to fluoroquinolones was 80.64%. We could access only one other paper from India evaluating microbiological resistance in patients with cirrhosis [19]. In that study the number of gram positive isolates was much higher (33%) and the incidence of ESBLs and Metallobetalactamases was lower but still quite high (60%) compared to western data. A paper published from Pakisthan [22] studied microbiological isolates but did not try and document resistance patterns. One may refer to other microbiological data in different clinical scenarios from different parts of India in this context.
The study conducted over 9 centers all over India and published in 2010 which evaluated resistance patterns of Gram negative bacteria isolated from intra-abdominal infections showed a preponderance of Klebsiella species and E. coli of which 70% and 79% respectively were ESBL producers. This was similar for both community acquired and hospital acquired infections. The incidence of Carbapenemase producers was much lower than in our study with 96% of isolates sensitive to imipenem [23]. Another study evaluating AmpC production in nosocomial Klebsiella showed that 32% were AmpC producers [24]. A study from Kolkata which investigated ESBL production in urinary isolates showed much lower rates of ESBL production among E. coli (31.6%) and Klebsiella (20.4%), with carbapenemase production being 4–10% [15].
As highlighted by Jalan et al. [1] the site of acquisition of infection is of prime importance as it determines the risk of multi drug resistant bacteria (MDR). Higher rates of MDR bacteria occur in infections acquired by the healthcare environment, quoted in their paper as 23–39% in nosocomial infections, 14–41% in HCA episodes and 0–16% in infections acquired in the community [1]. Fernandez et al.’s [9] study from Spain which included 669 infections from 2 series showed mortality of 25–48%, 9–23% and 7–21% respectively in NC, HCA and CA infections. They have shown infections with multi resistant bacteria are more common among nosocomial infections, have higher rates of treatment failure and associated septic shock (26% vs 10%) and higher hospital mortality (25% vs 12%) [9].
We noted a high level of fluoroquinolone resistance in our study. Fernandez et al. [9] also noted a high level of fluoroquinolone resistance in enterobacteriaceae which they attributed to a large number of patients being on norfloxacin prophylaxis. Our population, however, had very few patients on Norfloxacin prophylaxis. We attribute the high levels of resistance due to indiscriminate use of Fluoroquinolones in the community primarily as empirical treatment of acute diarrhea. We observe similar high levels of fluoroquinolone resistance in our non cirrhotic patients with UTI. Another important finding in our study is the preponderance of healthcare associated infections almost two thirds of our patients having either healthcare associated or nosocomial infections, whereas only one third only are community acquired. Among the 61 enterobacteriaceae isolated 17 (27.86%) were community acquired. Amongst these community acquired organisms only 6 (35.29%) showed no resistance. What was alarming was that 2 (11.76%) of community acquired enterobacteriaciae were carbapenemase producers.
The practical implications lie in initial antibiotic selection. The 2014 position paper from Jalan et al. [1] has recommended a 3rd generation cephalosporin(ceftriaxone), amoxicillin-clavulanate or fluoroquinolones (Levofloxacin, Ciprofloxacin or Moxifloxacin)for community acquired SBP, UTI, pneumonia and cellulitis and Piperacillin tazobactam or meropenem +/− glycopeptides in nosocomial infections for initial empiric therapy but have also stressed the fact that therapy needs to be tailored to local resistance patterns. Fernandez et al. in their paper [9] have discussed the problem with increasing resistance in nosocomial infections and have stressed that nosocomial SBP or SBE are now empirically treated with Tigecycline or a carbapenem (to cover ESBL-E); UTI associated with SIRS with a combination of a carbapenem and a glycopeptides (to cover ESBL-E and E. faecium); cellulitis with ceftazidime+glycopeptides (to cover MRSA and P. Aeroginosa). HCA and nosocomial pneumonia are now treated with carbapenems or Ceftazidime: plus Levofloxacin plus a Glycopeptide. The 2018 EASL guidelines [31] recommend third generation cephalsporins as empirical therapy in countries with low levels of bacterial resistance. However, Piperacillin/tazobactam is recommended in areas with low prevalence of MDR bacteria while Carbapenems are recommended for areas with high prevalence of ESBLs. Looking at our data we propose that empiric therapy with a suitable beta lactam/beta lactamase combination or cefepime may be considered as suitable choice for patients with community acquired or even health care associated infections. However HCA infections with SIRS or sepsis and nosocomial infections would require combination therapy with a group 2 carbapenem and a glycopeptide or Tigecycline as per local antibiotic sensitivity.
When we compared our group of patients who did not have any infection we found the 2 groups to be largely comparable in terms of age and baseline laboratory values such as hemoglobin. However the MELD score and CTP score were significantly lower in the control group. As a whole these were patients with less advanced liver disease and generally less ill than our infection group. Most of them were admitted either for paracentesis or GI bleeding. Only 1 patient in this group died during the index hospitalization.
Limitations of the present study may include the fact that this is a single center study; however similar data has been published from Barcelona [9]. We do not have long term follow up data of patients who survived the index episode of infection, as it has been pointed out that 1 year mortality among cirrhotic patients with infection may be as high as 63%. Our group of patients who did not have infection were not directly comparable to our main group as they represented patients with less advanced liver disease who were generally less unwell. This may be considered to further emphasize the fact that infection is a significant problem in this population. The fact that we were able to isolate micro-organisms from only 78 patients from our total population of 119 patients also represents a limitation of our study
5ConclusionInfections continue to be a major problem in patients with cirrhosis and they contribute significantly to increased mortality and morbidity. With the ongoing global crisis of antibiotic resistance it is becoming increasingly difficult to treat these infections promptly and adequately. Though the problem is a global problem the spectrum of infecting organisms and the pattern of sensitivity to antibiotics will necessarily show significant variability based on geographical location, prevalent antibiotic policies and pattern of antibiotic use. The importance of prompt initiation of appropriate antibiotic therapy has been shown to significantly improve outcomes and so more local guidelines based on more local data will need to be formulated to ensure improvement of outcomes.AbbreviationsCA Community Acquired Clinical and Laboratory Standards Institute Child Turcotte Pugh European Association for the Study of the Liver Extended Spectrum Beta Lactamases Health Care Associated Hepatitis B Virus Hepatitis C Virus International Normalised Ratio Lower Respiratory Tract Infection Multi Drug Resistant Model for End Stage Liver Disease Methicillin Resistant Staphylococcus aureus Methicillin Sensitive Staphylococcus aureus North American Consortium for End Stage Liver Disease Non Alcoholic Steatohepatitis Non Alcoholic Fatty Liver Disease Nosocomial Spontaneous Bacterial Peritonitis Urinary Tract Infections Ventilator Associated Pneumonia Vancomycin Resistant Enterococci Vancomycin Sensitive Enterococci white blood count
Asokananda Konar and Sujit Kar Purkayastha were responsible for conceptualisation and design of the study. Manisha Das Mandal was responsible for acquisition of data. Debkishore Gupta and Ajay Krishna Sarkar were responsible for microbiological studies and antibiotic strategies. Chandramouli Bhattacharya was responsible for analysis and interpretation of data. Asokananda Konar and Chandramouli Bhattacharya were responsible for writing the manuscript.
Confirmation of informed consentIt is confirmed that informed consent was obtained from all patients or their representative for publication of case details.
Conflict of interestThe authors have no conflicts of interest to declare.