Cirrhosis is an advanced stage of liver disease, compromising liver function with systemic health implications and poor quality of life. Hepatitis C virus (HCV) infection and alcoholic liver disease are the main causes of this pathology. However, since genetic factors may play a large role in the progression and severity of liver disease, and as apolipoprotein E (apoE) has been recognised to be mainly synthesised in the liver, apoE polymorphism studies are important to better understand the causal mechanisms in liver diseases. In this review, we summarise up-to-date studies addressing how apoE polymorphisms influence liver cirrhosis and liver transplantation outcomes and potential protective mechanisms. Although more clinical studies are needed to support these findings, the apoE ɛ4 allele seems to be protective against the progression of liver cirrhosis in the majority of aetiologies and the postoperative serum apoE phenotype of the transplanted subject receptors was converted to that of the donor, indicating that >90% of apoE in plasma is synthesised in the hepatic system.
Cirrhosis is the result of the advanced stage of liver disease, resulting from the replacement of the functional liver architecture with non-functional fibrotic tissue, being responsible for important health problems worldwide [1,2]. Infections by hepatitis C virus (HCV) and alcoholic liver disease are the main causes of liver cirrhosis [3,4]. However, this liver pathology can also be caused by other aetiologies such as metabolic diseases, hereditary (hemochromatosis, Wilson's disease, Alpha-1-antitrypsin deficiency), non-alcoholic steatohepatitis (NASH), autoimmune hepatitis, Budd-Chiari syndrome, primary biliary cirrhosis (PBC), and primary sclerosing cholangitis [1,2]. Therapeutic options include liver transplantation, which is the definitive treatment in patients with end-stage liver cirrhosis, improving the quality of life as well as the longevity of this population [1].
Human apolipoprotein E (apoE) consists of a 34kDa glycoprotein, containing 299 amino acid residues, which is an important protein component of very low density lipoproteins (VLDL) and a ligand for the low density lipoprotein receptor (LDL-R) [5–7].
ApoE is synthesised mainly in the liver, but also in the spleen, brain, kidney, lungs, adrenal gland, monocyte-macrophage, muscle tissues, central and peripheral nervous system. It has important actions in neuronal repair, in the regulation of lipid homeostasis, and in the transport and metabolism of triglycerides and cholesterol. It also has anti-inflammatory functions, skewing the pro-inflammatory macrophagic phenotype M1 to the anti-inflammatory M2 and decreasing the synthesis of interleukin-2 (IL-2), as well as roles in immunomodulatory activities such as the activation and proliferation of T lymphocytes [7–10].
Some studies have documented the association between apoE isoforms and diseases such as Alzheimer's disease, atherosclerosis, liver disease caused by HCV, human immunodeficiency virus (HIV) infection, HIV-associated dementia, pulmonary tuberculosis, childhood diarrhoea and herpes simplex virus infection [5,7,11,12].
ApoE was also evidenced in an experimental model as a sensitive marker in the graft function of transplanted hepatocytes, which is important since hepatocyte transplantation has been emphasised as a promising treatment for patients with liver or metabolic diseases and acute liver failure [13].
It is well documented that genetic factors play a key role in the severity and progression of liver diseases. Therefore, we set out to review the role of apoE polymorphisms in conditioning the natural history of pre- and post-transplant liver disease, as well as its association with the development of liver fibrosis and response to therapies.
2Polymorphism and different apolipoprotein e isoformsThe human apoE gene is polymorphic. In humans, this polymorphism is responsible for different apoE isoforms, due to amino acid substitutions at position 112 and 158 [5,14,15]. There are three common alleles of the apoE gene, which is located on chromosome 19 (19q13); these are called ɛ2, ɛ3 and ɛ4, with six possible genotypes: ɛ2/ɛ2, ɛ2/ɛ3, ɛ2/ɛ4, ɛ3/ɛ3, ɛ3/ɛ4, and ɛ4/ɛ4. The ɛ3 allele is the most frequent isoform, accounting for 70–80% of alleles worldwide, encoding the apoE3 isoform with a cysteine residue at position 112 and an arginine residue at position 158. The ɛ4 allele encodes the apoE4 isoform, with an arginine residue at both positions 112 and 158, whereas the ɛ2 allele has a point mutation causing the replacement of arginine for cysteine at position 158. These account for 10–15% (for the ɛ4 allele) and 5–10% (for the ɛ2 allele) of the alleles worldwide [5,7,9].
Previous studies have shown that the apoE ɛ4 allele may increase plasma triglyceride levels and decrease the levels of high density lipoprotein (HDL) cholesterol. In addition, it is associated with higher low density lipoprotein (LDL) cholesterol levels compared to the ɛ3 isoform. However, the ɛ2 isoform has a close association with hypertriglyceridaemia and hypocholesterolaemia [16]. Noteworthy, ∼15% of ɛ2/ɛ2 homozygous patients may develop familial dysbetalipoproteinaemia (also known as type III hyperlipoproteinaemia). This leads to hypercholesterolaemia and hypertriglyceridaemia and may be associated with obesity and insulin resistance [17,18].
3Apolipoprotein E and orthotopic liver transplantationOrthotopic liver transplantation is currently the most appropriate treatment for end stage liver disease, and may also influence the synthesis and degradation of genetically polymorphic-encoded plasma proteins. Thus, as apoE is a polymorphic protein in humans, some studies have already reported that it is possible to detect and quantify changes in this protein in patients who have undergone liver transplantation [9,15].
Linton et al. [15] performed a 29-patient sample study and reported that the postoperative serum apoE phenotype of the receptor was converted to that of the donor. This indicates that >90% of apoE in the plasma is synthesised in the hepatic system. On the other hand, there was no change in the apoE phenotype of cerebrospinal fluid (CSF) from the donor to the receptor phenotype after hepatic transplantation, indicating that most of the apoE in CSF is synthesised locally and not derived from plasma. Kraft et al. [9] also emphasised that more than 90% of apoE in humans is of hepatic origin, since the new apoE phenotype following liver transplantation corresponded to that of the donor organ.
In experimental models, serum apoE is a sensitive marker with which to monitor the functioning and survival of grafts of transplanted hepatocytes. These results are of significant importance, since hepatocyte transplantation may be a promising technique for patients with metabolic liver disease or acute liver failure [13]. In addition, by affecting lipoprotein metabolism, apoE polymorphisms may modulate the recurrence of HCV in individuals undergoing liver transplantation [19].
4Apolipoprotein E, hepatitis B and C viruses and hepatocellular carcinoma4.1Hepatitis B virusHepatitis B virus (HBV) infection is still very common (and worrying) in developing countries. Its aetiological agent is a DNA virus, hepatovirus of the family Hepadnaviridae; in particular, the long-standing infection can lead to liver failure, cirrhosis and hepatocellular carcinoma (HCC) [20–22].
Previous studies have documented the association between apoE genotypes and viral diseases, including herpes simplex virus (HSV), HIV, HCV, and HBV [21,23,24]. It has been documented that the ɛ3 allele of the apoE was frequent in patients with progressive HBV-related liver cirrhosis [25].
In addition, Shen et al. [21] enrolled 40 healthy volunteers and 199 patients with HBV, active hepatitis, severe hepatitis, cirrhosis and HCC, and demonstrated that serum levels of apoE ɛ3 progressively increased with disease severity (HBV carriers developed hepatitis, followed by cirrhosis and then ultimately HCC). The ɛ3 allele and ɛ3/ɛ3 genotype were the most prevalent in all subgroups. Furthermore, progressive serum elevation of interleukin-6 (IL-6) and a gradual decrease of IL-2 were associated with disease progression and severity, while apoE serum levels were positively correlated with serum IL-6 (but not with IL-2).
4.2Hepatitis C virusThe hepatitis C virus is a flavivirus and hepatotropic RNA virus, which can cause acute and chronic hepatitis C, as well as liver cirrhosis and hepatocellular carcinoma in humans [26]. However, there is a recent suggestion of a correlation between low cholesterol levels and infection by viruses C. Authors have reported that the mechanisms of HCV, HSV and HIV infection resemble each other, as all of these viruses compete with apoE to bind to cell receptors [23,27].
ApoE polymorphisms may be an important tool for monitoring the progression of fibrosis in patients with hepatitis C and normal alanine aminotransferase levels, as there may be competition mechanisms for viral entry and replication [27]. In other reports, HCV synthesised in individuals carriers of the apoE ɛ2 allele is associated with a low risk of infection and rapid elimination [28].
Pioneering studies by Wozniak et al. [24] have shown that the apoE ɛ4 allele was significantly more frequent in patients with chronic hepatitis C and mild liver disease compared to those with severe disease, indicating that the ɛ4 allele may be protective against liver injury due to HCV. Mueller et al. [29] also showed a significant low frequency of the apoE ɛ4 allele in patients diagnosed with chronic HCV infection, suggesting a protective action of this allele.
In the study by Price et al. [30] a significant lower frequency of both the ɛ2 and ɛ4 alleles was associated with a reduced infection risk in patients with HCV. Mueller et al. [31] also suggested a protective role of the ɛ4 allele and a higher risk of persistent HCV infection in ɛ3 allele carriers in chronically HCV-infected patients. Furthermore, a higher frequency of the ɛ4 allele was found among the non-cirrhotic chronic hepatitis C patients, supporting that the ɛ4 allele is protective against HCV infection [32]. In another study with 996 chronically HCV-infected patients, the apoE ɛ4 allele was poorly represented [31]. In addition, the same authors documented reduced viral loads in patients with the apoE ɛ4 allele who were chronically infected with HCV genotype type 1 [29], suggesting that this allele has a protective effect against HCV infection.
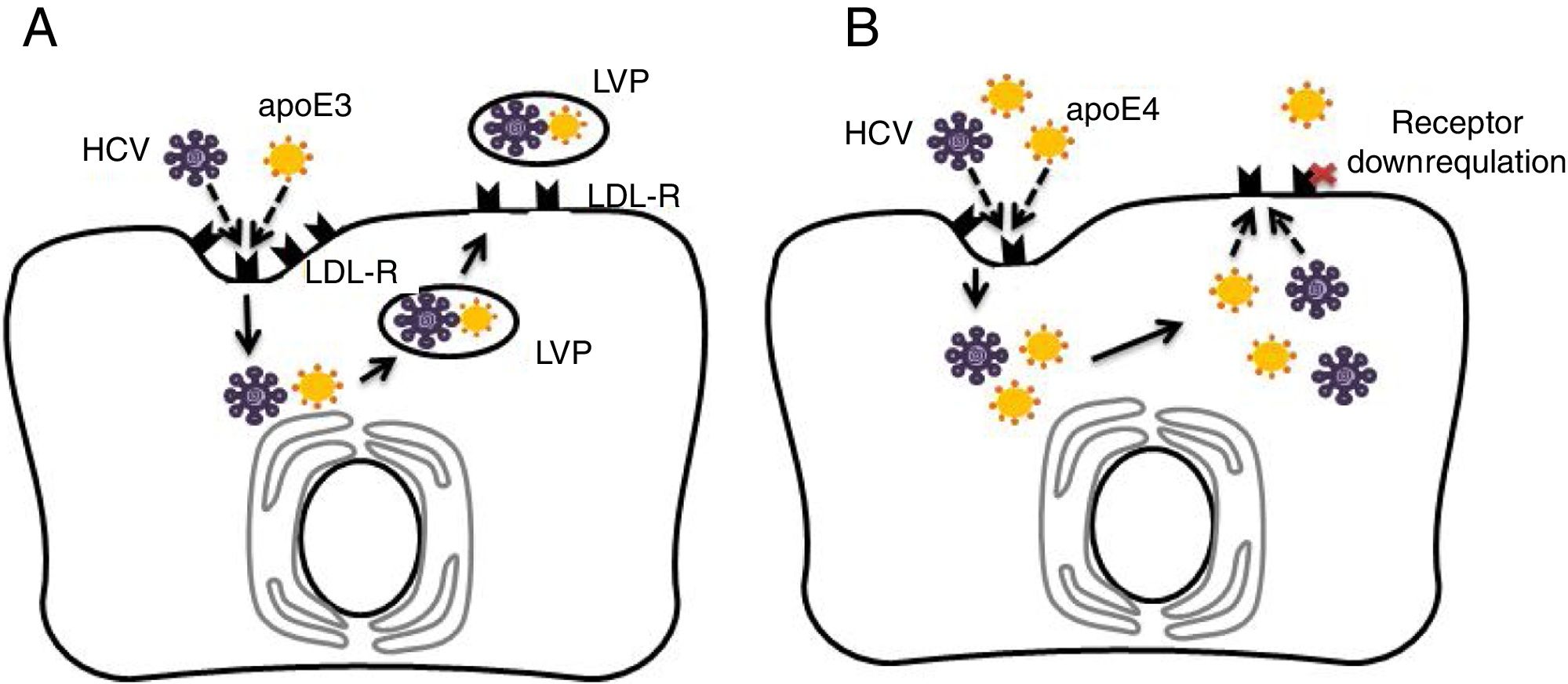
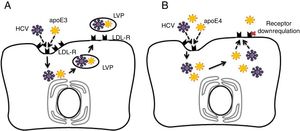
4.3Potential mechanisms for apoE4 protection against hepatitis C virus infectionAs discussed previously in our review, apoE4 has been suggested as a protective factor against HCV infection [33,34]. The entry of HCV into human hepatocytes is a multistep mechanism in which various host factors are involved, including LDL-R and heparan sulphate proteoglycans (HSPGs). The lipoviral particle (LVP), which is important for viral infectivity, initially binds LDL-R and HSPGs through apoE [35–37]. It has been recognised that the LDL-R is down-regulated in apoE4 carriers [23]. Thus, in apoE ɛ4 patients, the virus entry in hepatocytes may be reduced [10]. Fig. 1 depicts the potential protective mechanism of apoE ɛ4 against HCV in hepatocytes.
Comparison of apolipoprotein E3 (apoE3) and apolipoprotein E4 (apoE4) effects on hepatitis C virus (HCV) infection in hepatocytes. (A) Normal cycle of HCV entry in hepatocytes via low density lipoprotein receptor (LDL-R). HCV competes with apoE particles (apoE3 in this case) for binding of LDL-R, ending in the formation of lipo-viral-particle (LVP), which is important for virus infectivity. (B) ApoE4 proposed protection against HCV entry. In addition of apoE and virus competition for cell entry via LDL-R, the apoE4 carriers would show down-regulated LDL-receptors in hepatocytes and less viral capsuling.
ApoE would be the only specific factor for the production of infectious HCV particles; therefore, apoE could influence a late stage of virus infectivity after viral capsid envelopment, being essential for viral cell-to-cell transmission [38]. One hypotheses is that apoE ɛ4 indirectly affects LDL receptor expression by increasing the binding and internalisation of lipoproteins by apoE ɛ4 [31,39]. On the other hand, apoE ɛ4-induced hyperbetalipoproteinemia could directly interfere with uptake of the LDL-R mediated virus due to forced competition between free betalipoproteins and virus-lipoprotein particles for local LDL receptors [40]. Thus, the apoE4-related functional properties in lipid metabolism (and negative regulation of liver LDL receptors) may provide an explanation (corroborated with epidemiological evidence) of how apoE4 carriers are protected against HCV infection.
4.4Hepatocellular carcinomaHepatocellular carcinoma (HCC) is the most frequent primary liver cancer [41], and is mostly found in patients with liver cirrhosis, mainly due to infection by the hepatitis B and C viruses. Thus, as cirrhosis is the main risk factor associated with HCC, liver transplantation became the main treatment of this tumour [41–44].
Some studies have shown that apoE has antioxidant actions and is increased in malignant tumours such as gastric, prostate, ovarian and HCC, mainly due to the oxidative stress generated by the tumour cells [45–47]. Yokoyama et al. [48] found that apoE levels in tumour tissues were significantly higher than in normal non-tumour tissues in 88% of patients, without any increase in the plasma, and that apoE may be a histological marker for HCC. They also showed that 72.7% of patients with HCC were ɛ3/ɛ3.
Ahn et al. [25] found that the apoE plasma level was significantly higher in the group of patients with hepatic cirrhosis and the HCC group. They also observed that the ɛ3 allele and the ɛ3/ɛ3 genotype were the most frequent in both groups and that ɛ4 allele was the one that presented the lower probability of developing liver cirrhosis.
5Apolipoprotein E and primary biliary cirrhosisPrimary biliary cirrhosis (PBC) is an autoimmune cholestatic liver disease due to inflammation of small intrahepatic biliary ducts, which can result in fibrosis and cirrhosis of the liver in some patients; liver transplantation is the last therapeutic approach [49–51]. ApoE polymorphisms can modify the severity of PBC, acting on intestinal absorption and the excretion of bile salts. The phenotype may have an influence on the pathogenesis of PBC and also act on the disease response during treatment [50,51].
Corpechot et al. [50] reported that the ɛ3 allele and the ɛ3/ɛ3 genotype were the most frequent in PBC patients; however, there was no difference in both the disease and French control population, suggesting that ɛ3 is not a risk factor for the onset of PBC in the French population. Furthermore, after PBC treatment with ursodeoxycholic acid, among ɛ4 carriers, the liver enzymes did not return to baseline, suggesting a poor response to therapy in these patients. Conversely, in the study by Vuoristo et al. [51], the ɛ2 allele was seen significantly more often in patients with PBC, while the ɛ4 allele carriers showed better liver enzyme tests when treated with ursodeoxycholic acid. More studies with larger numbers are needed to better dissect these contradictory results.
6Apolipoprotein E and alcoholic liver cirrhosisAlcoholic liver cirrhosis due to chronic alcohol abuse has been a longstanding worldwide health problem, resulting in worrisome increasing in morbidity and mortality [52]. The incidence of the risk of death is higher in cirrhotic patients than the risk of developing liver cirrhosis, which indicates that low or moderate alcohol consumption is not related to significant increases in the risk of developing cirrhosis; however, this risk tends to increase exponentially from excessive alcohol intake [53]. In addition, not every individual who consumes alcohol regularly will develop alcoholic cirrhosis, as genetic and environmental factors also contribute to the development of this condition. However, it is important to emphasise that alcohol has an important effect on lipid metabolism, leading to hypertriglyceridaemia, hypercholesterolaemia and changes in lipoproteins [54,55].
However, previous studies have documented the influence of apoE polymorphisms in patients who developed hepatic cirrhosis due to alcohol [23,55]. In an experimental model, the authors observed that chronic long-term ingestion of ethanol in hepatic alcohol dehydrogenase-deficient deer mice led to the infiltration of T lymphocytes into the liver and hepatic steatosis. There was also a reduction in the frequency of lipid-carrying proteins, resulting in a decrease in the frequency of apolipoproteins in plasma [56].
In the analysis of patients with hyperlipidaemic or non-hyperlipidaemic alcoholic liver cirrhosis, Hernández-Nazara et al. [55] observed that the ɛ2 allele was closely associated with the hyperlipidaemic group and the early onset of cirrhosis, with alcohol intake <20 years, and that the allele ɛ4 was more frequent in the non-hyperlipidaemic group and apoE ɛ4 carriers were more resistant to cirrhosis with alcohol consumption >20 years. Iron et al. [54] studied alcoholic cirrhotic patients and observed that there was a higher frequency of ɛ4 and ɛ2 alleles. Frenzer et al. [57] did not identify a significant difference in apoE genotype frequencies when compared to alcoholic cirrhosis, pancreatitis, controls and blood donor groups. Furthermore, in the cirrhotic group, the ɛ3/ɛ3 genotype had a higher frequency, whereas ɛ4/ɛ4 was the least frequent.
7Non-alcoholic fatty liver disease or non-alcoholic steatohepatitisNon-alcoholic fatty liver disease (NAFLD) is characterised as the accumulation of excess liver fat and increased endogenous lipogenesis substances unrelated to chronic alcoholism [58,59]. Most of these patients may present with metabolic alterations such as dyslipidaemia, diabetes mellitus and obesity. In addition, they may develop non-alcoholic steatohepatitis (NASH), which may progress to fibrosis and cirrhosis [58,60]. ApoE is associated with different pathologies as well as with altered lipid profiles [61]. Previous studies with apoE deficient mice fed a cholesterol-rich diet have shown that this method may be a valuable alternative in NASH research [62]. Interestingly, ɛ4 allele patients had a significantly lower risk of NAFLD and a significant reduction in HDL cholesterol [63].
In another study, the ɛ3/ɛ3 genotype and ɛ3 allele were more prevalent in patients with NAFLD and also associated with increased risk of NASH, whereas the ɛ2/ɛ3 and ɛ3/ɛ4 genotypes and allele ɛ4 were associated with protection against NASH [64]. In addition, the ɛ2 allele and ɛ2/ɛ3 genotype have been shown protective against the development of NAFLD [65]. Conversely, other studies suggest that the apoE ɛ4 allele is a risk factor for NAFLD pathogenesis [66].
8Role of apolipoprotein E in liver fibrosisA protective effect of apoE ɛ4 against severe hepatic fibrosis has been supported by previous findings of lower ɛ4 allele frequency among patients with HCV-related severe hepatic fibrosis compared to those with mild liver disease [24]. Fabris et al. have reported a benefit of the apoE ɛ4 allele on the progression of fibrosis in liver transplant patients with recurrent hepatitis C [19]. However, in another study by Stachowska et al. [67] evaluating NAFLD, the ɛ4 allele was significantly associated with the development of advanced fibrosis due to disrupted hepatic fatty acid metabolism and increased 5-oxo-6,8,11,14-eicosatetraenoic acid production [66]. In addition, a study by Mueller et al. could not find an ɛ4 allele-protective effect against liver fibrosis in patients diagnosed with chronic HCV infection [29]. Nonetheless, the ɛ3/ɛ3 occurrence has been correlated with the rapid progression of fibrosis [27].
ApoE can facilitate cholesterol efflux from peripheral macrophages and other cells to form nascent discoidal high-density lipoprotein (HDL) after interaction with ABC transporters. The HDL particles deliver the cholesterol to hepatocytes via interaction with scavenger receptor B1 (SR-BI) or low-density lipoprotein (LDL) receptors. There is later conversion to cholesterol-bearing bile acids, therefore contributing to the reverse cholesterol transport from periphery to hepatocytes and to faecal excretion [68].
Hepatocytes can become steatotic by prolonged high-fat diets, indicating that hepatocytes may be overloaded by excess cholesterol delivery. Increased lipolysis (decreased β-oxidation of triglycerides) or increased de novo lipogenesis and reduced VLDL secretion from hepatocytes are also involved in this process [69]. ApoE has been recognised as an important contributing factor to improving liver steatosis. It has been shown that apoE-deficient mice chronically fed with western diets develop non-alcoholic steatohepatitis and liver fibrosis [62].
Chronic liver inflammation and liver steatosis may increase the rates of hepatocyte apoptosis and significantly increase the expression of platelet-derived growth factor BB (PDGF-BB) and transforming growth factor β (TGFβ) that lead to activation of Ito cells (hepatic stellate cells) and pro-fibrogenesis-activated genes [70]. Ito cells, which represent quiescent liver vitamin A-storing cells in the physiological state, are activated during chronic liver inflammation. These cells are transdifferentiated to myofibroblasts that produce extracellular matrix collagens and a myriad of inflammatory signals that lead to liver fibrosis and loss of vitamin A storage [71]. Interestingly, cultured Ito cells are able to synthesise and release apoE peptides [72].
It remains elusive whether apoE ɛ4 could be released during fibrosis and whether this peptide could improve this process.
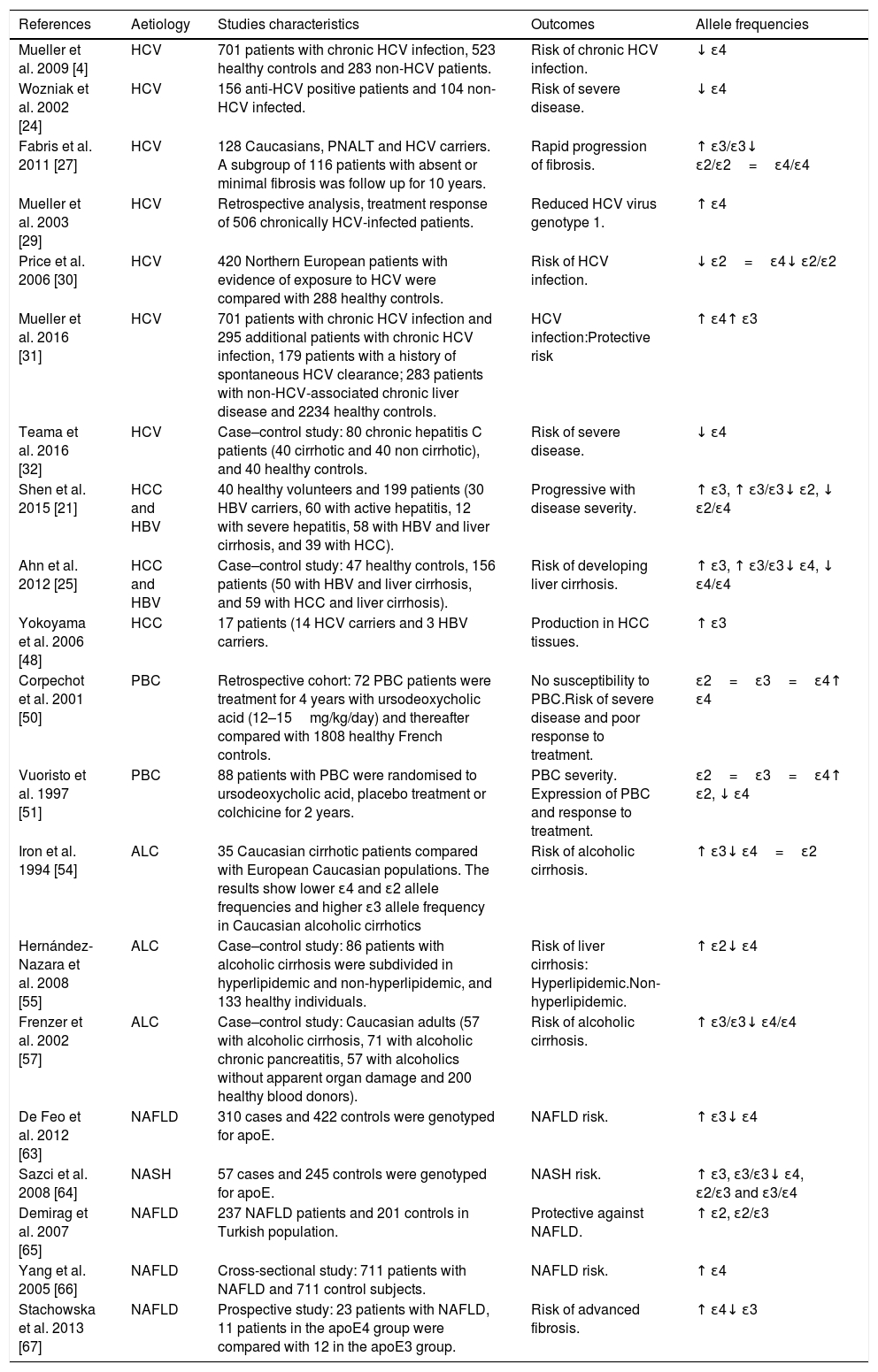
Table 1 summarises studies addressing apoE polymorphisms and liver diseases in clinical studies to date.
ApoE polymorphism studies on liver cirrhosis in humans.
| References | Aetiology | Studies characteristics | Outcomes | Allele frequencies |
|---|---|---|---|---|
| Mueller et al. 2009 [4] | HCV | 701 patients with chronic HCV infection, 523 healthy controls and 283 non-HCV patients. | Risk of chronic HCV infection. | ↓ ɛ4 |
| Wozniak et al. 2002 [24] | HCV | 156 anti-HCV positive patients and 104 non-HCV infected. | Risk of severe disease. | ↓ ɛ4 |
| Fabris et al. 2011 [27] | HCV | 128 Caucasians, PNALT and HCV carriers. A subgroup of 116 patients with absent or minimal fibrosis was follow up for 10 years. | Rapid progression of fibrosis. | ↑ ɛ3/ɛ3↓ ɛ2/ɛ2=ɛ4/ɛ4 |
| Mueller et al. 2003 [29] | HCV | Retrospective analysis, treatment response of 506 chronically HCV-infected patients. | Reduced HCV virus genotype 1. | ↑ ɛ4 |
| Price et al. 2006 [30] | HCV | 420 Northern European patients with evidence of exposure to HCV were compared with 288 healthy controls. | Risk of HCV infection. | ↓ ɛ2=ɛ4↓ ɛ2/ɛ2 |
| Mueller et al. 2016 [31] | HCV | 701 patients with chronic HCV infection and 295 additional patients with chronic HCV infection, 179 patients with a history of spontaneous HCV clearance; 283 patients with non-HCV-associated chronic liver disease and 2234 healthy controls. | HCV infection:Protective risk | ↑ ɛ4↑ ɛ3 |
| Teama et al. 2016 [32] | HCV | Case–control study: 80 chronic hepatitis C patients (40 cirrhotic and 40 non cirrhotic), and 40 healthy controls. | Risk of severe disease. | ↓ ɛ4 |
| Shen et al. 2015 [21] | HCC and HBV | 40 healthy volunteers and 199 patients (30 HBV carriers, 60 with active hepatitis, 12 with severe hepatitis, 58 with HBV and liver cirrhosis, and 39 with HCC). | Progressive with disease severity. | ↑ ɛ3, ↑ ɛ3/ɛ3↓ ɛ2, ↓ ɛ2/ɛ4 |
| Ahn et al. 2012 [25] | HCC and HBV | Case–control study: 47 healthy controls, 156 patients (50 with HBV and liver cirrhosis, and 59 with HCC and liver cirrhosis). | Risk of developing liver cirrhosis. | ↑ ɛ3, ↑ ɛ3/ɛ3↓ ɛ4, ↓ ɛ4/ɛ4 |
| Yokoyama et al. 2006 [48] | HCC | 17 patients (14 HCV carriers and 3 HBV carriers. | Production in HCC tissues. | ↑ ɛ3 |
| Corpechot et al. 2001 [50] | PBC | Retrospective cohort: 72 PBC patients were treatment for 4 years with ursodeoxycholic acid (12–15mg/kg/day) and thereafter compared with 1808 healthy French controls. | No susceptibility to PBC.Risk of severe disease and poor response to treatment. | ɛ2=ɛ3=ɛ4↑ ɛ4 |
| Vuoristo et al. 1997 [51] | PBC | 88 patients with PBC were randomised to ursodeoxycholic acid, placebo treatment or colchicine for 2 years. | PBC severity. Expression of PBC and response to treatment. | ɛ2=ɛ3=ɛ4↑ ɛ2, ↓ ɛ4 |
| Iron et al. 1994 [54] | ALC | 35 Caucasian cirrhotic patients compared with European Caucasian populations. The results show lower ɛ4 and ɛ2 allele frequencies and higher ɛ3 allele frequency in Caucasian alcoholic cirrhotics | Risk of alcoholic cirrhosis. | ↑ ɛ3↓ ɛ4=ɛ2 |
| Hernández-Nazara et al. 2008 [55] | ALC | Case–control study: 86 patients with alcoholic cirrhosis were subdivided in hyperlipidemic and non-hyperlipidemic, and 133 healthy individuals. | Risk of liver cirrhosis: Hyperlipidemic.Non-hyperlipidemic. | ↑ ɛ2↓ ɛ4 |
| Frenzer et al. 2002 [57] | ALC | Case–control study: Caucasian adults (57 with alcoholic cirrhosis, 71 with alcoholic chronic pancreatitis, 57 with alcoholics without apparent organ damage and 200 healthy blood donors). | Risk of alcoholic cirrhosis. | ↑ ɛ3/ɛ3↓ ɛ4/ɛ4 |
| De Feo et al. 2012 [63] | NAFLD | 310 cases and 422 controls were genotyped for apoE. | NAFLD risk. | ↑ ɛ3↓ ɛ4 |
| Sazci et al. 2008 [64] | NASH | 57 cases and 245 controls were genotyped for apoE. | NASH risk. | ↑ ɛ3, ɛ3/ɛ3↓ ɛ4, ɛ2/ɛ3 and ɛ3/ɛ4 |
| Demirag et al. 2007 [65] | NAFLD | 237 NAFLD patients and 201 controls in Turkish population. | Protective against NAFLD. | ↑ ɛ2, ɛ2/ɛ3 |
| Yang et al. 2005 [66] | NAFLD | Cross-sectional study: 711 patients with NAFLD and 711 control subjects. | NAFLD risk. | ↑ ɛ4 |
| Stachowska et al. 2013 [67] | NAFLD | Prospective study: 23 patients with NAFLD, 11 patients in the apoE4 group were compared with 12 in the apoE3 group. | Risk of advanced fibrosis. | ↑ ɛ4↓ ɛ3 |
Hepatitis C virus (HCV); Normal alanine aminotransferase levels (PNALT); Hepatocellular carcinoma (HCC); Hepatitis B virus (HBV); Primary biliary cirrhosis (PBC); Alcoholic liver cirrhosis (ALC); Non-alcoholic fatty liver disease (NAFLD); Increased allele frequency (↑); Decrease allele frequency (↓); Apolipoprotein (apoE).
Overall, this concise review has summarised accumulating evidence that apoE ɛ4 carriers are protected against chronic HCV infection, have slow progression of liver fibrosis, and are less likely to have alcoholic cirrhosis, NASH, HCC or HBV. On the other hand, apoE ɛ3 carriers are at higher risk for developing liver cirrhosis caused by NASH, NAFLD, HCC or HBV. We still can find contradictory results regarding the protective role of apoE ɛ4 on liver fibrosis and NAFLD. In addition, a gap of knowledge still exists regarding the apoE genotypes’ role on the mechanisms of liver injury following viral hepatitis. Interactional mechanisms of apoE with hepatitis viruses may be a potential target for gene-related therapies in the future.
Although further clinical studies are highly needed, in this essential review, we highlight that carriage of the apoE ɛ4 allele may exert a protective effect in reducing the progression of most liver diseases from different aetiologies.AbbreviationsHCV hepatitis C virus apolipoprotein E non-alcoholic steatohepatitis primary biliary cirrhosis very low density lipoprotein low density lipoprotein receptor interleukin-2 human immunodeficiency virus high density lipoprotein low density lipoprotein cerebrospinal fluid hepatitis B virus hepatocellular carcinoma herpes simplex virus interleukin-6 heparan sulfate proteoglycans lipoviral particle primary biliary cirrhosis non-alcoholic fatty liver disease scavenger receptor-B1 platelet-derived growth factor-BB transforming growth factor β
J.C.R.N., G.A.M., A.E.C.C.B.M, L.C.P., A.M.S., R.B.O., P.T. contributed to the study design and writing of the manuscript; J.C.R.N., R.B.O., P.T. critically revised the manuscript for important intellectual content and supervised the study.
Financial supportThe study was funded in part by the Brazilian funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Conflicts of interestThere are no conflicts of interest.