Hepatocellular carcinoma (HCC) is considered an immunogenic tumor that arises in chronically inflamed livers due to underlying chronic liver disease caused by viral and non-viral pathogenesis. This inflammation leads to tumor development and is associated to higher tumor immunogenicity.
For this reason immunotherapeutic approaches may be suitable therapeutic strategies for HCC. Indeed, several preclinical and clinical data support this hypothesis showing that immunotherapy and even more their combination may be a good alternative candidate for the treatment of HCC patients.
However, considering that the liver plays a central role in host defense as well as in the maintenance of self-tolerance, it is characterized by a strong intrinsic immune suppressive microenvironment as well as by a high immune evasion, which may represent a major impediment for an effective immune response against tumor. Furthermore, the low expression of tumor antigens on liver cancer cells leads to a lower T-cell activation and tumor infiltration, resulting in a less efficient control of the tumor growth and, consequently, in a worse clinical outcome.
For this reason, strategies should be developed to counteract the different factors in the HCC tumor microenvironment playing a major role in reducing the effects of immunotherapy.
Chronic inflammation and the intrinsic intra-hepatic immunosuppressive microenvironment occurring during liver fibrosis contribute to Hepatocellular carcinoma (HCC) development. Every year, more than 800 Mio people die from HCC worldwide making HCC the sixth most common neoplasm and the third leading cause of cancer death [1].
Therapies for HCC are dependent on the stage of disease. In the early stages, surgery represents the standard treatment with a 5-year survival rate in 70% of treated patients. When surgery or liver transplantation are not applicable, loco-regional therapies (i.e., radiofrequency, thermal and non-thermal ablation, and transarterial chemoembolization) represent a second line of therapy, with highly variable 3–5-year survival rates [2,3]. In advanced unresectable HCC, the only approved systemic therapies are represented by the tyrosine-kinase inhibitors sorafenib and regorafenib (first and second line treatment, respectively) as well as by the inhibitor of vascular endothelial growth factor receptors 1–3 lenvatinib (as second line treatment). However, such systemic therapies provide only a very limited survival benefit [4–6]. In addition, the systemic chemotherapy has been reported to be unsuccessful in HCC patients because of the intrinsic chemoresistence of hepatocytes as well as the related severe toxicities [7].
Furthermore, very few clinical trials reported encouraging results of low dose metronomic chemotherapy in HCC patients, which has been shown to have anti-immunosuppressive as well as immune-stimulating effects without relevant toxicity [8–10].
In such an adverse scenario, immunotherapeutic approaches may be suitable strategies for HCC, which is considered an immunogenic cancer because it arises in chronically inflamed liver [11].
In the last years several preliminary preclinical and clinical data have supported such an intrinsic immunologic characteristic of HCC. In particular, these studies have shown in the tumor microenvironment (HCC-TME) the dual suppressive and activating role of immune cells and the chemokine axis as well as the proinflammatory cytokines release, which contribute either to tumor eradication or to tumor progression [12]. And it is exactly the balancing of these two sides of the same coin that may determine the immunotherapy efficacy and therefore the tumor progression. In this framework, immunotherapeutic interventions, including cancer vaccines, may represent a novel and effective therapeutic tool for HCC in order to counterbalance the HCC-TME immunosuppression as well as to improve the activation of intra-tumor effector cells.
In this review, different immunotherapy approaches and their combinations for HCC are summarized.
2Tumor microenvironment in hepatocellular carcinomaThe liver is characterized by a strong intrinsic immune suppressive microenvironment which may represent a major barrier to an effective anti-tumor activity elicited by immunotherapeutic interventions.
The intrinsic immunologic composition of the liver plays a central role in host defense and even more in the maintenance of self-tolerance [13]. Several cells are involved in inducing such intra-hepatic tolerogenicity.
Liver sinusoidal endothelial cells (LSECs) represent the most potent scavenger system in the body, but have also an antigen-presenting cell (APC) function regulating the effector immune response in the liver [14]. In the physiological state, they prevent immune responses against bacterial antigens coming from the gut by inhibition of CD4+ and CD8+ T lymphocytes. Indeed, LSECs express high levels of the inhibitory molecule program death receptor ligand 1 (PD-L1) and low levels of the costimulatory molecules CD80 and CD86 on their surface [15,16]. Moreover, they also reduce the ability of dendritic cells (DCs) to activate T cells [17].
Similar to LSECs, Kupffer cells (KCs) promote immunological tolerance in the liver by removing from the circulation gut-derived materials as well as by producing inhibitory cytokines such as IL-10 and prostaglandins [18,19]. These cells have also a direct activation of inhibitory forkhead box P3 (FoxP3) in CD4+ T cells leading to proliferation of inhibitory CD4+ regulatory T cells (Tregs) [20,21].
Also the hepatic dendritic cells (HDCs) contribute to the tolerogenic microenvironment of the liver, being poor stimulators of effector CD4+ T cells. Indeed, they express low levels of MHC II and co-stimulatory molecules, exhibit low endocytotic ability, produce the anti-inflammatory prostaglandin (PG) E2 which, in turn, increases IL-10 secretion and induces Tregs cells [22].
Such a physiological immune suppressive microenvironment is even more marked during the formation and progression of HCC. A progressive and persistent downregulated immune gene profile has been identified during HCC progression, which leads to a lower tumor immunity in advanced stages of disease [23]. In the same study has been shown that the liver fibrotic state favors tumor progression, representing a physical barrier made by collagens and ECM proteins to prevent CD8+ CTL infiltration from peri-tumoral to intra-tumoral area. These observations suggest the possibility to use molecules able to disrupt the collagen/ECM structure in order to promote the intra-tumor infiltration by the CD8+ CTL trapped in the peritumoral zone [24].
Overall, the immune suppressive microenvironment is important to induce self-tolerance in the normal liver. However, this represents a strong obstacle for the development of an anti-tumor immunity as well as for the efficacy of immune-based therapeutic strategies. Therefore, approaches to modulate such unfavorable tumor microenvironment are needed in combination with immunotherapies for HCC.
3Immune evasion in hepatocellular carcinomaIn addition to the presence of cells with immune suppressive functions, the HCC TME is characterized by high expression of immune checkpoint molecules. The combination of the two leads to a markedly reduced activity of effector anti-tumor immune response and, consequently, to tumor immune evasion. Indeed, interaction between PD-1 and PD-L1 on tumor infiltrating lymphocytes and tumor cells, respectively, contributes to T cell exhaustion, tumor-specific T-cell dysfunction and immune evasion by cancer cells. Exhausted T cells express additional inhibitory molecules directly correlating with severity of exhaustion, which may reversed by combined PD-1/CTLA-4 blockade [25].
High expression of PD-L1 in HCC has been mainly found on Kupffer cells but also on tumor cells as well as on tumor infiltrating lymphocytes. Very often this is correlated with high PD-1 expression on CD8+ T cells and is associated with higher risk of cancer recurrence or metastasis and cancer-related death [26,27]. Additional inhibitory immune checkpoint molecules have been identified in HCC and correlated with poor prognosis. High expression of T-cell immunoglobulin-and mucin-domain-containing molecule-3 (Tim-3) and lymphocyte-activation gene 3 (LAG-3) have been identified on tumor infiltrating CD8+ T lymphocytes. In particular, Tim-3 is expressed only on T cells from tumor and not from the surrounding liver tissue. Moreover, it is expressed also on tumor-associated macrophages (TAM) [28].
The TIM-3 ligand Galectin-9 has been identified highly expressed on antigen presenting cells in HCC inducing T cell senescence and worse prognosis [29,30].
Similar to TIM-3, LAG-3 is expressed only on T cells from tumor and not from the surrounding liver tissue and treatment with specific blocking Abs has been shown to improve anti-tumor efficacy of anti-PD-1 blocking Abs [31].
In addition to the immune checkpoint pathway, also the chemokine axis plays a fundamental role in the tumor immune evasion, modulating the immune response in the TME and directly affecting HCC cell growth, invasion and migration properties. In particular, it has been demonstrated that in the HCC-TME the pro-inflammatory cytokines such as TNF, IFNG, and IL1 are significantly downregulated and are associated with increased levels of immunosuppressive cytokines (IL-4, IL-5, IL-8, and IL-10), thereby contributing to a higher aggressive tumor phenotype and poor prognosis [32,33]. Indeed, TNF and IFNG are involved in the activation of cytotoxic T lymphocytes to induce tumor killing, whereas elevated levels of IL4 and IL10 are associated with immune dysfunction and worse clinical outcome in cancer patients, including HCC [34–37].
4Mutational landscape and neoantigen in HCCThe efficacy of specific antitumor immune response is based not only on the right balance of effectors and immunosuppressive cells in the TME, but also on the ability of malignant cells to present tumor antigens to Antigen-presenting cells (APC), which will promote the infiltration of cytotoxic T cells in tumor site and activate their antitumor response [38,39]. The latter event is strongly associated with the intra-tumoral expression levels of tumor associated antigens (TAAs) as well as mutated antigens (neoantigens) [33,40–44]. However, considering that TAAs are not tumor specific and could be a sub-optimal target for cytotoxic T cells, the best non-self immunological target is represented by real tumor-specific antigens deriving from public or personal mutations in cancer cells [45]. Indeed, infiltration of cytotoxic T cells into the TME has been reported to be directly correlated to tumor mutational burden (TMB) resulting in a better clinical response [44,46–48].
Given that the TMB is strongly related to the intra-tumor load of neoantigens, the latter represent a predictive biomarker for clinical response to immunotherapy treatment with immune checkpoint inhibitors (ICIs). Indeed, recent studies have shown that efficacy of ICIs correlates not only with the level of immune infiltration (“hot” or “cold” tumors) but also with the number of predicted neoantigens target of the infiltrating T cells [49–52].
Among others, HCC ranks as a medium variable tumor, with an average mutational burden of 5 somatic mutations per Mb, corresponding to approximately 60 non-synonymous substitutions within expressed genes, leading to generation of neoantigens targeted by tumor-infiltrating T cells [53].
Identification of naturally presented neoantigens on the surface of tumor cells by high sensitivity mass spectrometry, has proven to be much more cumbersome than the one of TAAs and needs to be improved for a broader application [54–56].
Currently, tumor neoantigens are predicted and validated through bioinformatics and experimental pipelines for which a general consensus has not achieved yet. Indeed, a simple prediction step is not sufficient for identifying meaningful neoantigens which need to meet several parameters. The predicted affinity value to HLA molecules of neoantigen should be lower than 50nM and greater than 10 times compared to the affinity of the corresponding wild type epitope (differential agretopicity index, DAI>10). In addition, neoantigens should not share sequence homology with any wild type cellular self antigens, implying that a very limited fraction of cancer mutations give rise to mutated antigens which are immunologically relevant [48,57–60]. Neoantigens with such characteristics have been classified as “alternatively defined neopeptides” (ADNs) by Rech et al. [60] Furthermore, if such neoantigens show homology with pathogen-derived epitopes, the pre-existing pathogen-specific immunity will respond faster and stronger to such neoantigens, resulting in a more efficient control of the tumor evolution and, consequently, in a better clinical outcome [58,60].
The only neoantigen discovery in HCC has been recently reported in a study by our group [48]. Neoantigens were classified as “true predicted neo-antigens” (TPNAs) only when their corresponding wt peptides showed a low prediction ranking in NetMHCStabPan. Moreover, TPNAs showed no or low sequence homology with corresponding wt peptides in the 4 aa residues of the epitope facing the TCR (p1, p4, p5, p8). Finally, when TPNAs showed homology with pathogen-derived antigens, HCC patients showed a significantly improved survival [48].
The overall results show that the quality more than the quantity of neoantigens expressed by cancer cells may predict clinical outcome as well as guide selection of the most appropriate target antigens for cancer immunotherapy.
5Immunotherapy strategies for HCCIn view of the above, the immunotherapy strategies in HCC should be able to counteract the different mechanisms that underlie the HCC-TME, such as the immunosuppressive and immune evasion in TME, the effector T cell dysfunctions, the alterations in immune checkpoint molecules expression and deregulation of cytokine profiles. In this regards, therapeutic strategies based on immune modulatory strategies (e.g. chemotherapy and anti-checkpoint molecules), or active immunization with cancer vaccines, or their combination could be highly effective.
To date a limited number of immunotherapy trials for HCC have been conducted with yet modest results.
5.1Immunotherapy trials based on Immune checkpoint inhibitorsTo date only CTLA-4 and PD-1/PD-L1 inhibitors have been evaluated in HCC in four clinical trials. In particular, the anti-CTLA-4 Tremelimumab, was the first molecule to be clinically evaluated for safety and tumor response in HCC [61,62]. The phase II clinical trial involved 20 HCC patients infected with hepatitis C virus and not eligible for surgery or locoregional therapies, who were treated with a suboptimal dose of the MAb. Three of the 17 evaluable patients showed partial response (PR) (17.6%) and an additional 10 patients (58.8%) were found to have stable disease (SD). Time to progression was 6.48 months and the overall survival reached 8.2 months. Decreased viral load was shown, suggesting an antiviral effect of immune checkpoint blockade and possible usefulness in patient with HCC related to viral etiology.
Subsequently, a phase I/II study by Duffy et al. described the potential of standard treatments (i.e. tumor ablation utilizing RFA and TACE) to enhance the efficacy of Tremelimumab, improving the infiltration of intratumoral effector CD8+ T cells [63].
The last two clinical trials, based on anti-PD-1/PD-L1 blockade, have achieved more encouraging results.
An open-label phase I/II clinical trial has been conducted to evaluate the anti-PD-1 nivolumab in HCC patients with various etiologies irrespective of any previous treatment with sorafenib. Primary endopoints were safety, immunogenicity and antitumor activity (CheckMate 040). Complete response (CR) was reported in 3/214 (1.4%) patients and partial response (PR) in 39/214 (18.2%) patients, with a 83% overall survival (OS) at 6 months. Adverse events of grade 3–4 were observed in 25% of the study population [62]. More recently, very comparable results were observed in the KEYNOTE-224 trial which evaluated the anti-PD-1 Pembrolizumab in HCC patients who had progressed on sorafenib [64]. Complete response (CR) was reported in 1/104 (1%) patients and partial response (PR) in 17/104 (16%) patients, with a 54% overall survival (OS) at 12 months. Currently ongoing is a global phase III randomized control trial which is comparing nivolumab with sorafenib as first-line treatment in patients with advanced HCC (CheckMate 459) [ClinicalTrials.gov identifier: NCT02576509]. The overall safety profile of checkpoint inhibitors in HCC patients is manageable. Indeed, although HCC patients are very often characterized by liver dysfunction due to the underlying liver chronic disease, the substantial increase of AST and ALT induced by treatment with checkpoint inhibitors was not so relevant to cause discontinuation [65].
5.2Active immunotherapy approaches: cancer vaccine strategiesSimilar to ICIs clinical trials, also the number of cancer vaccine clinical trials in HCC are very limited showing only some degree of efficacy. A restricted number of TAAs has been identified in HCC and variable T cell response to them has been previously assessed [66]. Moreover, most of them are not specific to HCC and only few have been targeted in cancer vaccine clinical trials [67].
The first clinical trial was conducted in the early 2000’, based on an alpha fetoprotein (AFP) derived peptide which induced a specific peptide T-cell response [68]. Vaccination with the same peptides presented by autologous DCs loaded ex vivo did not improve the results [69]. Subsequently, two vaccine approaches based on autologous DCs pulsed ex vivo either with a lysate of the autologous tumor or of the HepG2 cell line, have shown limited improvements in clinical outcomes [70–72].
An open label phase II clinical trial based on telomerase peptide did not lead to any complete or partial responses in advanced HCC patients [73], and similar results were reported in a phase I trial evaluating a GPC3 peptide [74].
A very innovative strategy is currently pursued for identification of shared “off-the-shelf” HCC-specific antigens within the HEPAVAC project (www.hepavac.eu) [75]. Novel HCC-associated antigens have been identified and a multi-epitope, multi-HLA peptide vaccine has been produced which is currently under evaluation for safety and immunogenicity in early-intermediate stage HCC patients undergoing surgical and/or loco-regional treatments (NCT03203005). The vaccination protocol will include also an actively personalized vaccine (APVAC) in a subset of vaccinees, based on patient-specific HCC neoantigens.
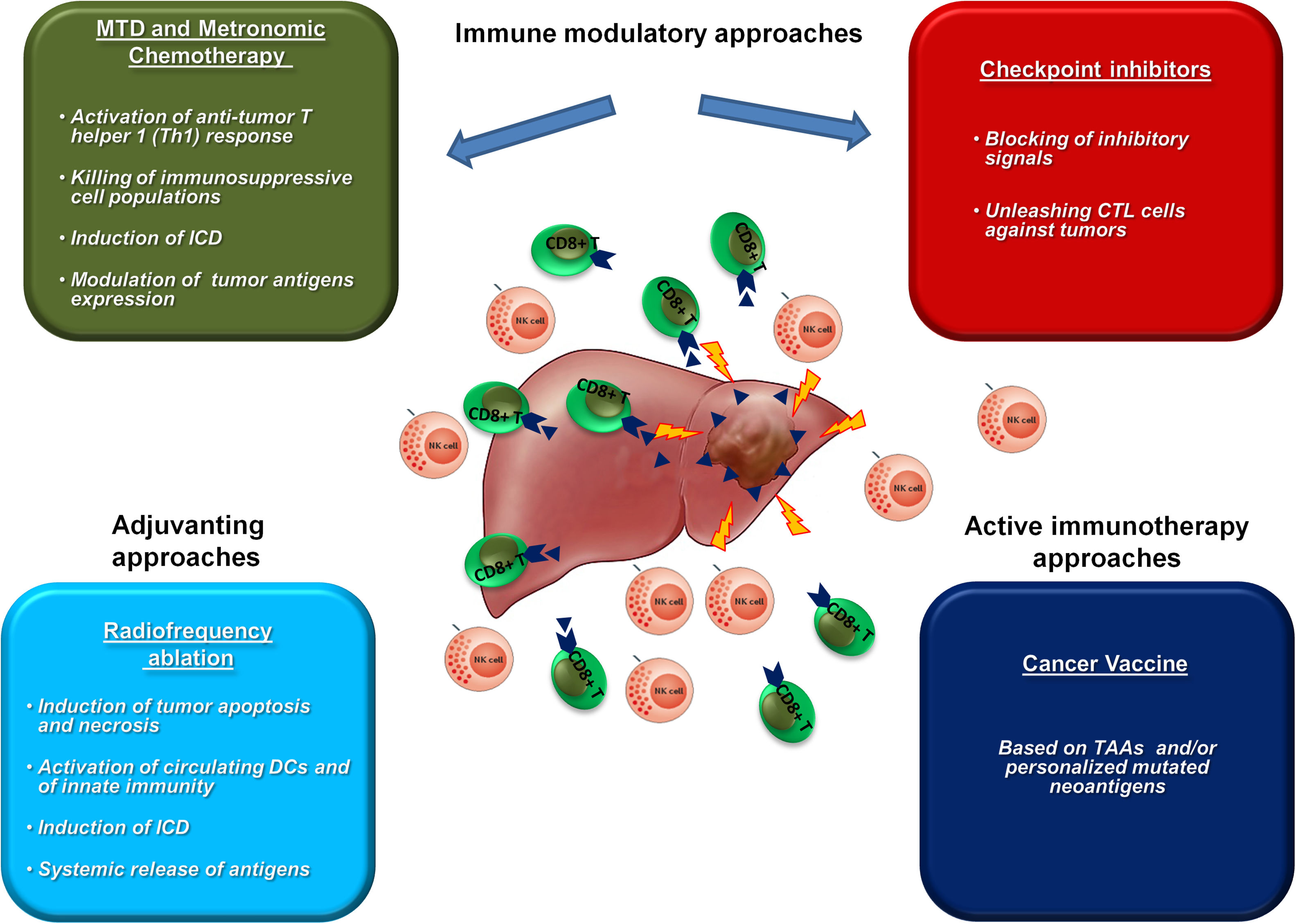
6Immune modulatory approaches in HCCThe efficacy of immunotherapies in HCC can be significantly improved by combination with immunomodulatory approaches able to positively modulate the TME aiming at counterbalancing the strong immune suppressive setting (Fig. 1).
Chemotherapy is considered immune suppressive, however maximum tolerate dose (MTD) as well as low-dose metronomic chemotherapy may enhance antitumor immunity by several mechanisms. In particular, several studies have demonstrated not only the selective killing of immunosuppressive cell populations (e.g. MDSCs and Tregs), but also the induction of immunogenic cell death (ICD) in cancer cells with release of danger signals able to polarize DCs and activate an anti-tumor T helper 1 (Th1) response. Moreover, they can modulate the expression of tumor antigens and immune checkpoint molecules, modifying the TME and improving the efficacy of immunotherapy treatments [76–81].
Radiofrequency ablation (RFA) is considered the first line treatment option in early stage HCC patients not suitable for surgical therapies, resulting in tumor destruction by induction of tumor apoptosis and necrosis [82,83]. The release of TAAs as well as neoantigens induces significant intratumoral immune infiltrates and activation of immune response [84–87].
For the above reasons, such immunomodulatory treatments may significantly improve the efficacy of anticancer immune responses induced by cancer vaccines.
Several clinical trials have shown a better clinical outcome compared to individual treatments [88,89]. In particular, cytotoxic drugs can improve anti-tumor effects of cancer vaccines counteracting the immune-suppression, enhancing cross-presentation of tumor antigens and increasing the number of effector cells in the tumor microenvironment [90–94].
Systemic treatment with low-dose cyclophosphamide in patients with advanced HCC has been shown to be safe and to decrease the frequency and suppressor function of circulating regulatory T cells in peripheral blood, unmasking α-fetoprotein-specific CD4+ T-cell responses [95]. However, a combination of such a treatment with a cancer vaccine based on hTERT peptide (GV1001) did not show antitumor efficacy in respect to tumor response and time-to-progression [73].
Combination of RFA and cancer vaccine has been evaluated in pre-clinical experimental settings, showing a significant enhancement of antitumor immunity with local and distal tumor regression [96,97]. A single clinical trial has shown that combination of RFA and a GPC3 peptide vaccine improved the 1-y recurrence rate in HCC patients with GPC3-positive tumors, compared to RFA alone [98].
Along the immunomodulatory effect of treatments, ICIs release the brake on the immune response, unleashing cytotoxic T cells against tumors, even those elicited by a cancer vaccine. Several pre-clinical studies have investigated combination strategies including cancer vaccines and checkpoint inhibitors, all of them showing significant enhancement of anti-tumor response associated with increased infiltration of effector CD8+ T cell [94,99,100].
Combination of vaccines and immune checkpoint inhibitors has been evaluated in clinical trials in different cancer settings showing priming, expansion and boosting of an effective tumor immunotherapy [101–103]. None of such combinatorial strategies have been evaluated in HCC yet.
7ConclusionsAlternative treatments, such as immunotherapies, are needed for HCC. Preliminary clinical trials with ICIs show a great potential in HCC as first and second line treatment. In addition, novel active immunotherapies (e.g. cancer vaccines) are currently developed and evaluated in clinical trials based on new TAAs as well as personalized mutated neoantigens.
Combination strategies including chemotherapy, RFA or checkpoint inhibitors together vaccines have been evaluated in several pre-clinical settings and in handful number of clinical trials. The latter strategy is predicted to be expanded in HCC in the coming years.
FundingThis work was supported by the EU FP7 Project Cancer Vaccine development for Hepatocellular Carcinoma – HEPAVAC (Grant Nr. 602893); Transcan2 – HEPAMUT project; Italian Ministry of Health through Institutional “Ricerca Corrente” (LB). AP and MT are funded by HEPAVAC. AM is funded by “Ricerca Corrente”.
Competing interestsThe authors declare no potential conflicts of interest.