Immune reconstitution syndrome is a recognized complication with initiation of highly active antiretroviral therapy for acquired immune deficiency syndrome patients co-infected with hepatitis B. Hepatitis B flares are seen in 20%-25% of patients after initiation of highly active antiretroviral therapy, an estimated 1%-5% of whom develop clinical hepatitis. We present a case of highly active antiretroviral therapy initiation for HIV that led to a flare of HBV activity despite antiviral therapy directed towards both. Liver biopsy and longitudinal serologic evaluation lend support to the hypothesis that the flare in activity was representative of IRIS. Importantly, we document eAg/eAb seroconversion with the IRIS phenomenon.
Hepatitis B virus (HBV) infection is common with an estimated 2 billion people having evidence of past or present infection.1 While the United States is considered a low prevalence region of the world, 0.3% of Americans are Hepatitis B surface antigen (HBsAg) positive—translating to 800,000 with active infection.2 Partly due to shared modes of transmission with Human Immunodeficiency Virus (HIV), an estimated 10% of HIV patients are co-infected with HBV.3 Those who acquire HIV/HBV concomitantly have a significantly higher risk to progression of chronic hepatitis B-21% vs. 7%.4 Hepatitis Be antigen (HBeAg) to antibody (HBeAb) seroconversion rates are significantly lower in coinfected populations.5 In addition, HIV/HBV co-infected patients experience more rapid progression of fibrosis and subsequent complications including cirrhosis, hepatocellular carcinoma and need for liver transplantation.6 Such rapid progression is evident even in patients with lower HBV viral loads and alanine aminotransferase (ALT) levels.7 Current guidelines recommend treatment of all co-infected patients with antiviral therapy targeting both HIV and HBV.8
Immune Reconstitution Syndrome (IRIS) is a recognized complication with initiation of highly active antiretroviral therapy (HAART) for acquired immune deficiency syndrome (AIDS) in HIV/HBV patients. HBV flares are seen in 20-25% of patients after initiation of HAART, an estimated 1-5% of whom develop clinical hepatitis.9 In this report, we present a case of HAART initiation for HIV that led to a flare of HBV activity despite antiviral therapy directed towards both. Liver biopsy and longitudinal serologic evaluation lend support to the hypothesis that the flare in activity was representative of IRIS. Importantly, we document eAg/eAb seroconversion with this phenomenon.
CaseA 57-year-old male originally from Nigeria presented with a 2-week history of right upper quadrant abdominal pain, nausea, fatigue and confusion. Medical history was significant for a recent diagnosis of HIV/HBV co-infection 2 months prior, identified when he presented with ataxia attributed to neurotoxoplasmosis. At that time, labs were notable for low CD4 count, positive HIV and HBV viral loads, and normal AST/ALT consistent with AIDS and immune tolerant, chronic HBV (Table 1). Hepatitis C antibody was negative. He was treated for Toxoplasmosis with pyrimethamine, leucovorin and clindamycin as well as initiated on HAART with emtricitabine, tenofovir and reltegravir. Sulfamethoxazole-trimethoprim was also started for pneumocystis jiroveci pneumonia prophylaxis. He remained on therapy for 2 months without incident; with outpatient, labs showing normal aminotransferases at 1 month of therapy.
Viral serologies.
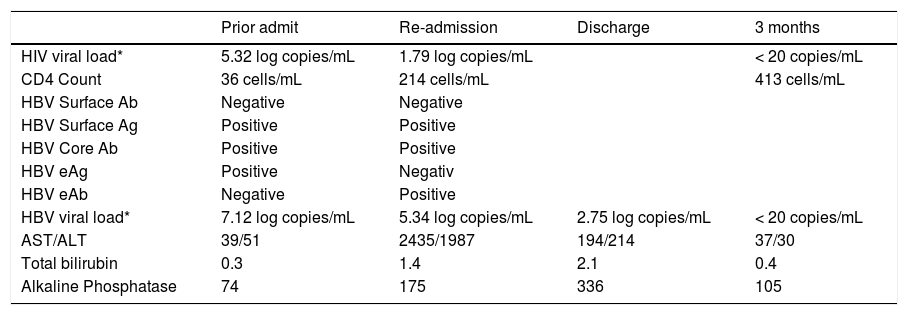
| Prior admit | Re-admission | Discharge | 3 months | |
|---|---|---|---|---|
| HIV viral load* | 5.32 log copies/mL | 1.79 log copies/mL | < 20 copies/mL | |
| CD4 Count | 36 cells/mL | 214 cells/mL | 413 cells/mL | |
| HBV Surface Ab | Negative | Negative | ||
| HBV Surface Ag | Positive | Positive | ||
| HBV Core Ab | Positive | Positive | ||
| HBV eAg | Positive | Negativ | ||
| HBV eAb | Negative | Positive | ||
| HBV viral load* | 7.12 log copies/mL | 5.34 log copies/mL | 2.75 log copies/mL | < 20 copies/mL |
| AST/ALT | 39/51 | 2435/1987 | 194/214 | 37/30 |
| Total bilirubin | 0.3 | 1.4 | 2.1 | 0.4 |
| Alkaline Phosphatase | 74 | 175 | 336 | 105 |
On re-presentation, labs were notable for significantly elevated AST/ALT consistent with a hepatocellular type liver injury pattern despite improvements in both HIV and HBV viral loads (Table 1). There was also evidence of HBV eAg to eAb seroconversion. HBV genotyping revealed genotype E with C1858T precore mutation but no polymerase or basal core promotor mutations. The patient was admitted for further workup and management. An abdominal ultrasound and CT scan revealed no focal lesions, nodularity or fatty infiltration of the liver. A repeat MRI showed almost complete resolution of his brain lesions from toxoplasmosis. Toxicology was consulted to help determine the etiology of the patient’s liver injury. Given the temporal association with the initiation of multiple new medications, drug induced liver injury was initially considered likely. Sulfamethoxazole-trimethoprim and azithromycin were determined to be the most likely culprits, although the patient had no rash, fever, lymphadenopathy or eosinophilia typical of a hypersensitivity reaction from the former agent. Infectious disease and hepatology were also consulted and his antiviral therapy was continued while is prophylaxis medications were held. The significant transaminitis only minimally improved however and on hospital day 7, a liver biopsy was performed.
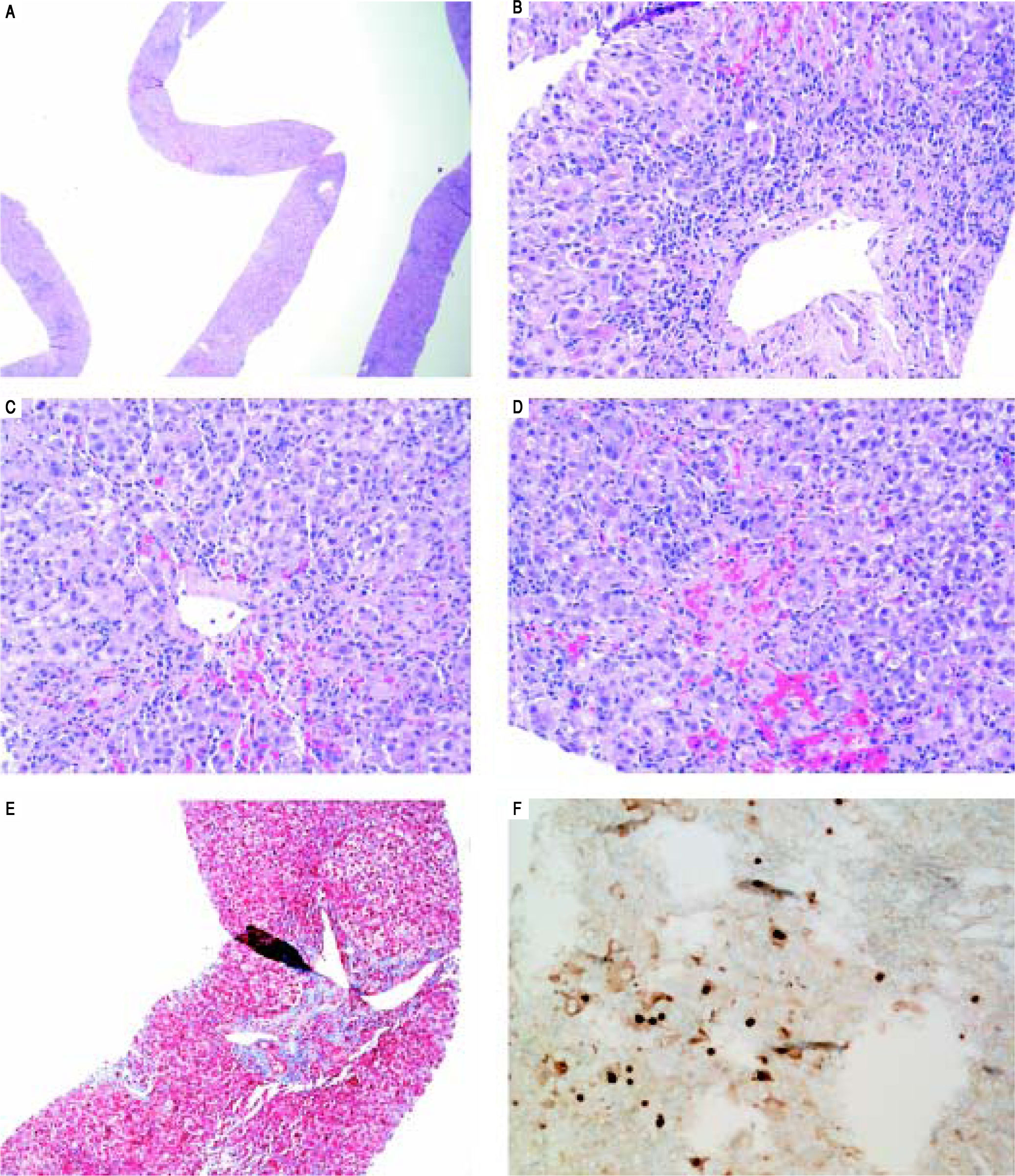
On H&E stain, moderate to severe active hepatitis with central zone dropout, focal periportal hepatocyte dropout of mild to moderate degree, and diffuse lobular activity with mixed inflammatory infiltrates and cellular necrosis were seen (Figures 1A-Figures 1D). There was mild periportal fibrosis on trichrome stain (Figures 1E). Typical of HBV infection, HBcAg staining showed multiple positive nuclear straining and significant focal cytoplasmic staining (Figure 1F). There were rare HBsAg staining and no evidence of drug toxicity.
Liver biopsy. A. Low power view showing active hepatitis with disarray and inflammation. B. Portal tract with mixed infiltrate, interface activity and hepatocyte injury (swelling and apoptosis), C-D. Lobular activity and pericentral inflammation with hepatocyte dropout, hemorrhage, and cholestasis. Plasma cells are present. E. Trichrome stain showing mild porta fibrosis. F. Hepatitis B core antigen stain with nuclear staining and significant focal cytoplasmic staining.
The patient’s clinical, laboratory and histological findings suggested HBV-related IRIS. HAART was continued and appropriate toxoplasmosis and prophylaxis therapy were restarted. He was discharged on hospital day 12 after symptoms and transaminitis improved significantly. Subsequently, AST/ALT levels normalized and HIV and HBV viral loads (were undetectable 3 months after discharge (Table 1).
DiscussionIRIS is an inflammatory disorder associated with paradoxical worsening of a pre-existing infection, either previously recognized or occult.10 The condition is commonly seen in AIDS patients although transplant patients may be affected. Diagnostic features include AIDS with low CD4 count; positive virologic/immunological response to HAART; clinical manifestations of an inflammatory condition; temporal association between initiation of HAART and onset; absence of other causes such as drug reactions, noncompliance or ineffective HAART therapy and other infection.11 The most common associated GI pathogen is HBV. Typical onset is 2-8 weeks after initiation of HAART. Symptoms include B-symptoms, anorexia, nausea, fatigue, tender hepatomegaly and jaundice. IRIS is usually self-limiting and HAART and HBV treatment are usually continued.9
The molecular mechanism of liver injury in IRIS appears to be different than for injury in non-HIV infected patients. Injury in mono-infected patients is caused by immune-mediated damage of HBV-infected hepatocytes. Multiple cytokines are implicated including tumor necrosis factor (TNF)-a, interferon (IFN)-y and activated natural killer (NK) cells. Ultimately both HBV-specific and predominately nonspecific CD8+ T cells present to the liver and cause damage to hepatocytes.12 The IRIS immune response is associated with recruitment of monocytes and CD8 + T cells to the liver but is not thought to involve NK cell-mediated immunity.13
Our case is nicely illustrative IRIS/flare of HBV activity with imitation of HAART. Serial viral PCR showed clear evidence of effective treatment of both his HIV and HBV with decreasing viral loads. Serum markers at presentation were consistent with immune tolerance with minimally elevated AST/ALT and positive HBeAg. At representation, injury associated with immune clearance was seen: increasing AST/ALT, eAg/eAb seroconversion, and decreasing viral loads. Interestingly, no similar flare of his neurologic symptoms to suggest toxoplasmosis related IRIS which has also been described.14 The patient was continued on HAART including tenofovir throughout his course and improved with symptomatic management. Interestingly, our patient had genotype E HBV with C1858T precore mutation, found almost exclusively in Africa. In one study from India, HIV/HBV co-infected patients had different profiles of mutations than mono-infected patients.15 To the best of our knowledge phenotypic diversity has not been studied in genotype E patients.
We found only one other study which addressed the features of HIV/HBV related IRIS on liver biopsy.16Two patients’ biopsies showed evidence of mild lobular hepatitis and mild lymphocytic inflammation which was diffusely positive for CD3 and CD8. These patients were both asymptomatic and had milder elevations of AST/ALT levels compared to our patient. eAg/eAb seroconversion was not documented in these cases. However, histological findings were similar and point to IRIS as the causative process instead of worsening HBV infection or drug toxicity.
While HBV related IRIS is relatively common, AST/ ALT levels rarely reach >10 times the normal limit.17 Frank decompensated liver failure is even rarer, typically limited to patients with advanced cirrhosis.18 IRIS related hepatic flare has been shown to rapidly lower HBV DNA and HBsAg levels.19 We postulate that the severity of the patient’s hepatic flare was ultimately beneficial in rapid clearance of HBV and development of HBe antibodies which is much less common in HIV/HBV patients.
The diagnosis of IRIS can be challenging. Hepatotoxicity with HAART medications is common but criteria for IRIS include ruling out drug toxicity. Given the known associated hepatotoxicity of HIV medications, treatment is sometimes stopped or adjusted in patients with worsening liver function. However, patients are at risk for severe worsening HBV infection with cessation of treatment. Given the very low likelihood of acute liver failure, continuing HAART and HBV treatment is most often appropriate. It is therefore important that providers have a high index of suspicion for IRIS in newly treated HIV/HBV co-infection and manage it appropriately.
Abbreviations- •
Ab: antibody.
- •
Ag: antigen.
- •
AIDS: acquired immune deficiency syndrome.
- •
ALT: alanine aminotransferase.
- •
HAART: highly active antiretroviral therapy.
- •
HBeAb: hepatitis Be antibody.
- •
HBeAg: hepatitis Be antigen.
- •
HBsAg: hepatitis B surface antigen.
- •
HBV: hepatitis B virus.
- •
IRIS: immune reconstitution syndrome.
The authors declares that there is no conflict of interest regarding the publication of this article.