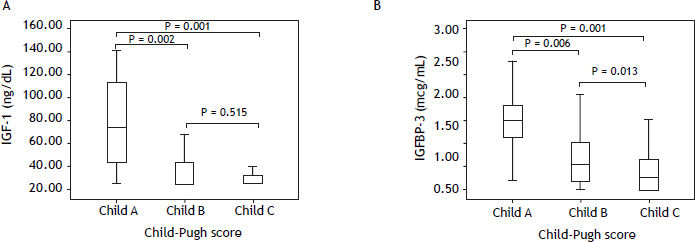Background. IGF-I and IGFBP-3 are part of IGF system and, due to their predominantly hepatic synthesis, they seem to correlate with hepatic dysfunction intensity. Aims. To investigate the significance of IGF-I and IGFBP-3 in patients with decompensated liver disease.
Material and methods. Cross-sectional study that included cirrhotic patients admitted to hospital due to complications of the disease, in whom IGF-I and IGFBP-3 serum levels were measured by chemiluminescence. Results. Seventy-four subjects with a mean age of 53.1 ± 11.6 years were included in the study, 73% were males. IGF-I levels were positively correlated with IGFBP-3 and albumin, and negatively correlated with Child-Pugh, MELD, creatinine, INR and aPTT ratio. IGFBP-3 levels were positively correlated with IGF-I and albumin, and negatively correlated with Child-Pugh, MELD, creatinine, INR, total bilirubin and aPTT ratio. Significantly lower scores of IGF-I and IGFBP-3 were observed in patients with higher MELD values and higher Child-Pugh classes (P < 0.05).
Conclusions. In cirrhotic patients admitted to hospital due to complications of the disease, IGF-I and IGFBP-3 serum levels were associated with variables related to liver dysfunction and to more advanced liver disease. The levels of these markers seem to undergo little influence from other clinical and laboratory variables, therefore mainly reflecting hepatic functional status.
Cirrhosis is the end stage of various chronic liver diseases and is characterized by a diffuse hepatic involvement, fibrosis, necrosis, and regenerative nodules. Several clinical manifestations can be observed as a result of portal hypertension and impaired liver synthesis capacity. In general, the natural course of cirrhosis is characterized by an asymptomatic phase, also known as compensated phase, followed by a rapidly progressive phase characterized by specific complications and also known as decompensated cirrhosis.1 The transition from compensa- ted forms of the disease to decompensated cirrhosis occurs at a rate 5–7% per year.2 Once patients have developed the first episode of decompensation, complications tend to accumulate and life expectancy is markedly reduced, with a median survival of two years.2
Patients with cirrhosis characteristically show a broad spectrum of metabolic disorders, including malnutrition, insulin resistance, osteopenia, and hypogonadism.3–5 These complications are at least in part linked to changes in the GH-IGF axis observed as a result of liver disease. The growth hormone (GH) is a peptide hormone released from the anterior pituitary that stimulates growth, cell reproduction and regeneration, exerting metabolic effects on bone, cartilage, fat, muscle, heart, immune system, and others.6,7 In the liver, GH activation of GH receptors induces the Insulin-like growth factor-I (IGF-I) gene transcription, and subsequently the synthesis and release of IGF-I to the plasma.8 IFG-I is the major form of insulin-like growth factors (IGFs) family and it is thought to exert anabolic effects on the amino acid and carbohydrate metabolism, increasing muscle mass and improving bone mineral content and intestinal barrier function.8 IGFBP-3 is the most important component of circulating binding proteins of the IGF system and it is mostly produced by the endothelial lining and Kupffer cells in the liver.9,10
As a reflection of decreased hepatic synthetic function, lower IGF-I and IGFBP-3 serum levels have been demonstrated in patients with cirrhosis when compared to healthy controls.7,11 These markers also seem to be related to the degree of hepatic dysfunction, as decreased levels were reported in more advanced stages of cirrhosis.7,11 Therefore, these tests appear to represent promising tools for evaluating patients with liver cirrhosis. New markers of hepatic synthetic function would be especially useful in evaluating patients admitted to the hospital due to complications of cirrhosis, who have high mortality and for whom fast therapeutic decisions are critical. The aim of this study was to investigate the significance of IGF-I and IGFBP-3 serum levels in patients admitted to the hospital due to complications of cirrhosis.
Material and MethodsPatientsCross-sectional observational study that included consecutive adult subjects (≥ 18 years of age), admitted at the Emergency Unit of a Brazilian tertiary-care hospital due to complications of the disease (ascites, hepatic encephalopathy, upper gastrointestinal bleeding secondary to portal hypertension, and hepatorenal syndrome). Patients with decompensated cirrhosis hospitalized due to infectious diseases were also considered eligible. Patients in the following situations were excluded: admissions for elective procedures or not related to complications of cirrhosis, doubtful diagnosis of liver cirrhosis, and refusal or inability of the patient or caregiver to understand the terms of the informed consent.
The diagnosis of cirrhosis was established either histologically (when available) or by the combination of clinical, imaging and laboratory findings in patients with evidence of portal hypertension. In the case of more than one hospital admission during the study period, only the most recent hospitalization was considered.
The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration and was approved by our institutional review board.
MethodsPatients were evaluated within 24 h of admission by one of the researchers involved in the study, and the following clinical variables were collected: age, sex, race, smoking history, etiology of cirrhosis, history of previous decompensations, and the presence of ascites. Current alcohol intake was defined as an average overall consumption of 21 drinks per week or more for men and 14 drinks per week or more for women during the 4 weeks before enrolment (one standard drink is equal to 12 g absolute alcohol).12 All subjects underwent laboratory evaluation at admission, and the following tests were performed for this study: sodium, creatinine, albumin, total bilirubin, international normalized ratio (INR) and ratio of activated partial thromboplastin time (aPTT ratio). All individuals with suspected infection at admission where investigated according to clinical judgment of the attending physician.
Hepatic encephalopathy was graded according to the West Haven criteria,13 and the liver disease severity at admission was assessed by the Child-Pugh classification14 and by the MELD score (calculated with the laboratory tests performed at admission).15
IGF-I and IGFBP-3 serum levelsIGF-I and IGFBP-3 serum levels were measured in serum samples collected at admission and stored at −20 °C. The tests were performed within one week of collection. Serum levels of IGF-I and IGFBP-3 were measured by immunochemiluminescence (Immulite® 2000, Diagnostic Products Corp., Los Angeles, CA). The reported analytic sensitivity of this assay is 25 ng/dL for IGF-I and 0.50 mcg/mL for IGFBP-3.
Statistical analysisThe normality of the variable distribution was determined by One-Sample Kolmogorov-Smirnov test. The correlation between the numerical variables was evaluated by the Spearman’s correlation coefficient. To compare IGF-I and IGFBP-3 levels according to the Child-Pugh classification and the different MELD tertiles, we initially used the Kruskal-Wallis test followed by the Mann-Whitney U test for comparison between two subgroups. The other comparisons were performed using the Mann-Whitney U test. A P value of less than 0.05 was considered statistically significant. Statistical analysis was performed by SPSS software version 17.0 (SPSS Inc., Chicago, IL).
ResultsCharacteristics of the SampleOne hundred and one admissions due to complications of liver cirrhosis were reported between January and November 2011. When evaluated based only on the most recent hospitalization, a total of 74 individuals composed the final sample of the study.
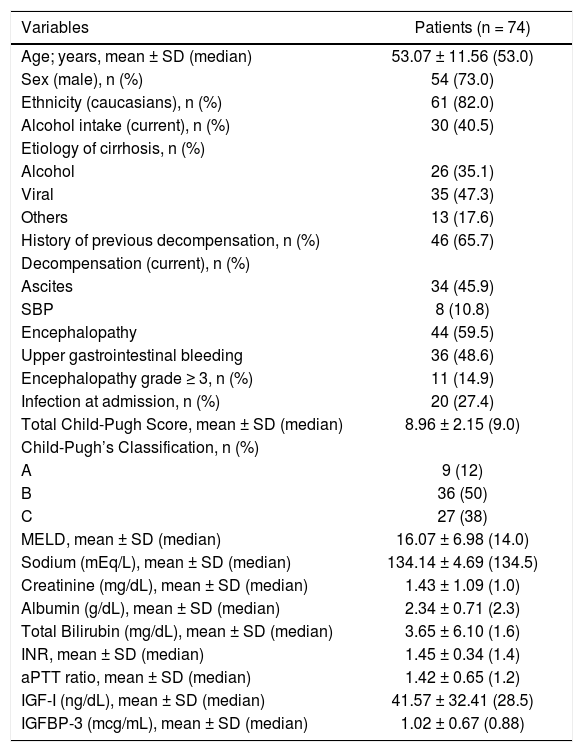
Table 1 exhibits the characteristics of the included patients. The mean age was 53.07 ± 11.56 years, 82.0% were Caucasians, and a male predominance was observed (73.0%). Previous history of cirrhosis decompensation was observed in 65.7% of the sample and 40.5% of subjects reported alcohol intake in the previous 30 days. Regarding the etiology of cirrhosis, chronic viral hepatitis was reported in 47.3% of the cases (HBV infection in 5.4% and HCV in 41.9%), alcohol in 35.1%, and other less common causes in 17.6% of the sample.
Demographic, clinical, and biochemical features of included patients.
| Variables | Patients (n = 74) |
|---|---|
| Age; years, mean ± SD (median) | 53.07 ± 11.56 (53.0) |
| Sex (male), n (%) | 54 (73.0) |
| Ethnicity (caucasians), n (%) | 61 (82.0) |
| Alcohol intake (current), n (%) | 30 (40.5) |
| Etiology of cirrhosis, n (%) | |
| Alcohol | 26 (35.1) |
| Viral | 35 (47.3) |
| Others | 13 (17.6) |
| History of previous decompensation, n (%) | 46 (65.7) |
| Decompensation (current), n (%) | |
| Ascites | 34 (45.9) |
| SBP | 8 (10.8) |
| Encephalopathy | 44 (59.5) |
| Upper gastrointestinal bleeding | 36 (48.6) |
| Encephalopathy grade ≥ 3, n (%) | 11 (14.9) |
| Infection at admission, n (%) | 20 (27.4) |
| Total Child-Pugh Score, mean ± SD (median) | 8.96 ± 2.15 (9.0) |
| Child-Pugh’s Classification, n (%) | |
| A | 9 (12) |
| B | 36 (50) |
| C | 27 (38) |
| MELD, mean ± SD (median) | 16.07 ± 6.98 (14.0) |
| Sodium (mEq/L), mean ± SD (median) | 134.14 ± 4.69 (134.5) |
| Creatinine (mg/dL), mean ± SD (median) | 1.43 ± 1.09 (1.0) |
| Albumin (g/dL), mean ± SD (median) | 2.34 ± 0.71 (2.3) |
| Total Bilirubin (mg/dL), mean ± SD (median) | 3.65 ± 6.10 (1.6) |
| INR, mean ± SD (median) | 1.45 ± 0.34 (1.4) |
| aPTT ratio, mean ± SD (median) | 1.42 ± 0.65 (1.2) |
| IGF-I (ng/dL), mean ± SD (median) | 41.57 ± 32.41 (28.5) |
| IGFBP-3 (mcg/mL), mean ± SD (median) | 1.02 ± 0.67 (0.88) |
SD: standard deviation. SBP: spontaneous bacterial peritonitis. MELD: model for end-stage liver disease. INR: international normalized ratio. aPTT ratio: ratio of activated partial thromboplastin time.
Upon admission, upper gastrointestinal bleeding was observed in 48.6% of cases, ascites in 45.9%, hepatic encephalopathy in 59.5%, and spontaneous bacterial peritonitis in 10.8%. Hepatic encephalopathy grade ≥ 3 was present in 14.9% of the patients. When evaluated according to the Child-Pugh classification, 12% were classified as stage A, 50% stage B, and 38% stage C. The mean MELD score was 16.07 ± 6.98 (median 14.0).
Relationship between IGF-I and IGFBP-3 levels and the studied variablesData on laboratory variables are shown in table 1. The mean levels of IGF-I were 41.57 ± 32.41 ng/ dL (median of 28.5 ng/dL) and of IGFBP-3 were 1.02 ± 0.67 mcg/mL (median of 0.88 mcg/mL).
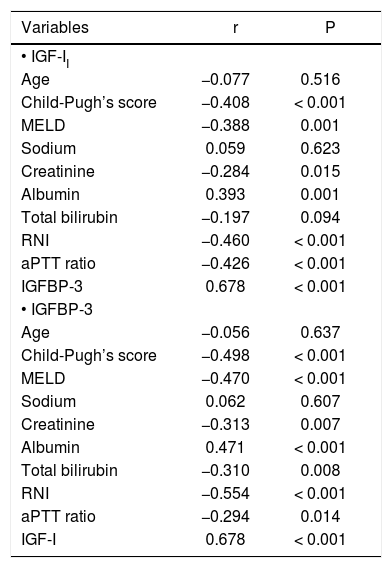
When the correlation between IGF-I levels on admission and the other numerical variables was studied, a positive correlation between this marker and the levels of IGFBP-3 (r = 0.678, P < 0.001) and albumin (r = 0.393, P = 0.001) was observed. A negative correlation was observed between IGF-I levels and the total Child-Pugh score (r = −0.408, P < 0.001), MELD score (r = −0.388, P = 0.001), creatinine (r = -0.284, P = 0.015), INR (r = −0.460, P < 0.001) and aPTT ratio (r = −0.426, P < 0.001). No significant correlations were observed between IGF-I and age, sodium, and total bilirubin.
Regarding IGFBP-3, a positive correlation between this marker and IGF-I (r = 0.678, P < 0.001) and albumin (r = 0.471, P < 0.001) was observed. A negative correlation was observed between IGFBP-3 levels and the total Child-Pugh score (r = −0.498, P < 0.001), MELD score (r = −0.470, P < 0.001), creatinine (r = -0.313 P = 0.007), INR (r = −0.554, P < 0.001), total bilirubin (r = −0.310, P = 0.008) and aPTT ratio (r = −0.294, P = 0.014). No significant correlations were observed between IGFBP-3 and age or sodium. Table 2 shows the correlation coefficients between IGF-I and IGFBP-3 levels and the other numeric variables.
Spearman’s correlation coefficient between IGF-I and IGFBP-3 levels and the numeric variables.
| Variables | r | P |
|---|---|---|
| • IGF-II | ||
| Age | −0.077 | 0.516 |
| Child-Pugh’s score | −0.408 | < 0.001 |
| MELD | −0.388 | 0.001 |
| Sodium | 0.059 | 0.623 |
| Creatinine | −0.284 | 0.015 |
| Albumin | 0.393 | 0.001 |
| Total bilirubin | −0.197 | 0.094 |
| RNI | −0.460 | < 0.001 |
| aPTT ratio | −0.426 | < 0.001 |
| IGFBP-3 | 0.678 | < 0.001 |
| • IGFBP-3 | ||
| Age | −0.056 | 0.637 |
| Child-Pugh’s score | −0.498 | < 0.001 |
| MELD | −0.470 | < 0.001 |
| Sodium | 0.062 | 0.607 |
| Creatinine | −0.313 | 0.007 |
| Albumin | 0.471 | < 0.001 |
| Total bilirubin | −0.310 | 0.008 |
| RNI | −0.554 | < 0.001 |
| aPTT ratio | −0.294 | 0.014 |
| IGF-I | 0.678 | < 0.001 |
MELD: model for end-stage liver disease. INR: international normalized ratio. aPTT ratio: ratio of activated partial thromboplastin time.
When IGF-I levels were evaluated according to specific complications of cirrhosis observed at admission, no associations with the presence of ascites or gastrointestinal bleeding (P > 0.05) were found. Those individuals with hepatic encephalopathy at admission had lower IGF-I levels compared to the other patients (25.0 vs. 33.9 ng/dL, P = 0.037). Concerning IGFBP-3 levels, subjects with ascites on admission showed significantly lower median values of this marker when compared to the rest of the patients (0.65 vs. 1.08 mcg/mL, P = 0.003). Similarly, lower median IGFBP-3 was also observed in those patients with hepatic encephalopathy on admission (0.78 vs. 1.18 mcg/mL; P = 0.013). No differences in IGFBP-3 levels were observed when subjects with upper gastrointestinal bleeding were compared to the other patients.
IGF-I and IGFBP-3 levels were also related to other clinical variables of interest. No significant differences in IGF-I and IGFBP-3 levels were observed when the patients were compared according sex, etiology of cirrhosis (viral vs. non-viral), diabetes mellitus, current smoking, and current alcohol intake. Individuals with diagnosis of infection at admission showed lower median IGFBP-3 (0.72 vs. 1.10 mcg/mL, P = 0.009) and a trend toward lower median IGF-I (25.0 vs. 31.7 ng/dL, P = 0.057) when compared to the remaining patients.
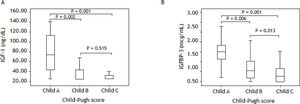
IGF-I and IGFBP-3 serum levels according to the degree of liver dysfunctionTable 3 shows IGF-I and IGFBP-3 levels according to the Child-Pugh’s classification. Significantly lower IGF-I levels were observed among those with higher Child-Pugh scores (P = 0.001). When comparing the different subgroups, higher IGF-I levels were observed in patients classified as Child-Pugh A as compared to patients classified as Child-Pugh B (P = 0.002) or C (P = 0.001). No differences were observed when comparing IGF-I levels between Child-Pugh B and C patients (P = 0.151) (Figure 1A). Similarly, individuals in higher stages of the Child-Pugh’s score exhibited lower IGFBP-3 levels as compared to the remaining patients (P < 0.001). When comparing the subgroups, individuals classified as Child-Pugh A had significantly higher levels of IGFBP-3 than those rated as Child-Pugh class B (P = 0.006) or C (P < 0.001). IGFBP-3 levels were also higher among those classified as Child-Pugh B as compared to Child-Pugh C (P = 0.013) (Figure 1B).
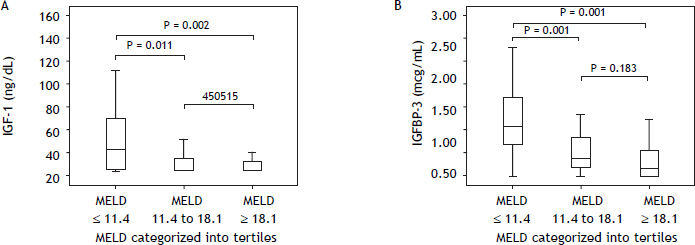
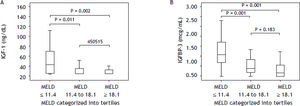
Table 4 shows IGF-I and IGFBP-3 levels according to the MELD score categorized into tertiles. Significantly lower IGF-I concentrations were observed among patients with higher MELD scores (P = 0.004) (Figure 2A). When comparing the subgroups, significantly higher IGF-I levels were observed in the subgroup with MELD ≤ 11.4 as compared to patients with MELD scores between 11.4 and 18.1 (P = 0.011) or ≥ 18.1 (P = 0.002). IGF-I levels did not differ when comparing patients with MELD between 11.4 and 18.1 in relation to those with scores ≥ 18.1 (P = 0.450) (Figure 2A). Similar results were observed for IGFBP-3 levels, which were significantly decreased in individuals with higher MELD scores (P < 0.001). In the subgroup comparison, individuals classified in the lowest MELD tertile showed higher concentrations of IGFBP-3 in relation to patients classified in the intermediate (P = 0.001) or higher tertiles (P < 0.001). No differences in IGFBP-3 levels were observed for comparison of individuals classified in the intermediate tertile and those in the upper tertile (P = 0.183) (Figure 2B).
The approach and management of cirrhotic patients in emergency departments are hampered not only by the heterogeneity of the underlying liver disease itself, but also by multiple complications usually observed in this setting. A close relationship between the intensity of liver dysfunction and prognosis of patients with cirrhosis is described.1. For that reason, the identification of markers of hepatic synthetic function, especially objective biochemical tests, could allow a better assessment of these individuals in emergency situations. Ideally, these tests should be inexpensive, reproducible, readily available and undergo little influence from other factors not related to liver synthesis capacity.
IGF-I is a peptide that acts as a mediator of the GH and stimulates somatic growth, inducing an anabolic response on tissues. It is synthesized by multiple mesenchymal-type cells, primarily in the liver. IGFBP-3 is the most abundant of six high affinity-binding proteins that share a common function in regulating the bioavailability of the insulin-like growth factors. It circulates in a saturated state and undergoes the influence of the same variables that affect serum concentrations of IGF-I.16–18 Given the importance of the liver in the synthesis and action of the IGF system components, conditions that impair liver function might result in reduced serum levels of IGF-I and its ligands.
In the present study, significant correlations were observed between IGF-I serum levels and other variables directly or indirectly associated with the intensity of liver dysfunction, such as albumin, INR, and creatinine. Similar results were observed in a Turkish study that included 42 patients with cirrhosis, in which IGF-I levels were positively correlated with albumin and sodium, and negatively correlated with serum creatinine.7 The negative correlation between IGF-I and creatinine observed in both studies probably reflects the severity of cirrhosis and not the glomerular filtration rate itself, since chronic kidney disease has been associated with higher levels of IGF-I.19 In another study investigating 34 subjects receiving outpatient treatment for cirrhosis, Donaghy, et al. demonstrated a positive correlation between IGF-I and albumin, but not with INR.20 However, this study included a relatively small number of patients, with a lower proportion of individuals classified as Child-Pugh B or C, which may explain the differences observed.
IGF-I levels were also evaluated according to the Child-Pugh’s Classification and MELD scores. Lower levels of this marker were observed in individuals at higher stages of the Child-Pugh’s classification or with higher MELD scores. Similar results were observed by Wu, et al., that reported significantly lower IGF-1 levels in cirrhotic patients when compared to the control group and, more importantly, among cirrhotic patients, those with a more severe liver dysfunction (Child-Pugh C) had the lowest concentrations of this marker.11 More recently, Assy, et al. demonstrated that cirrhotic patients in more advanced stages of the Child-Pugh’s classification or with higher MELD scores not only have lower levels of IGF-I, but also exhibit lower increase of this peptide in response to GH stimulation.21 Furthermore, the authors showed that IGF-I levels, whether basal or in response to GH administration, seem to reflect the extent of hepatocellular dysfunction and not the intensity of portal hypertension or the nutritional status.21
In the present study, IGFBP-3 levels also correlated with variables related to liver function such as albumin, total bilirubin, INR, and creatinine. These results are consistent with previous studies that evaluated the correlation between IGFBP-3 and markers of hepatic synthetic function.7,20,22 Similarly, as discussed above for IGF-I, the correlation observed between IGFBP-3 levels and creatinine probably reflects the severity of the underlying liver disease, since previous studies indicate that IGFBP-3 levels does not seem to be influenced by renal function.23
When IGFBP-3 levels were evaluated according to Child-Pugh’s classification and MELD score, we observed that individuals with evidence of more advanced liver disease had lower concentrations of this marker. Similar results were first published by Donaghy, et al. who investigated 35 patients with liver cirrhosis and reported lower IGFBP-3 levels in Child-Pugh C patients as compared to the remaining participants.20 After this study, other authors published similar results, reinforcing the hypothesis that IGFBP-3 concentrations seem to reflect the severity of liver disease in cirrhotic patients.7,11,24–26 In a recent study, Rehem and El-Shikh reported that IGFBP-3 and IGF-I together with Child-Pugh score is more effective in predicting hepatic dysfunction and its severity than Child-Pugh score alone. In addition, these markers were significantly lower in hepatocellular carcinoma patients than in both healthy subjects and in subjects with liver cirrhosis.27
Serum levels of IGF-I and IGFBP-3 may be influenced by several conditions not related to the liver, as well as by specific individual characteristics and lifestyle habits, such as cigarette smoking, alcohol consumption.8 Although not all conditions that could potentially alter the serum levels of these tests have been systematically evaluated, no significant associations with variables such as age, sex, smoking and alcohol intake were observed. These findings suggest that the influence of non-hepatic factors seems to be small, offering even more support to the use of IGF-I and IGFBP-3 in the assessment of liver synthesis capacity.
Lower IGF-I and IGFBP-3 levels were observed in subjects with a diagnosis of infection at admission when compared to those without infectious complications. Although, there are few studies regarding the influence of infection on IGF-I and IGFBP-3 serum levels, experimental data suggests the association between low IGF-I levels and a higher rate of bacterial translocation.28 Other authors have suggested a relationship between IGF-I levels and chronic infectious diseases, such as HIV infection.29 However, in the present study, it is likely that the lower IGF-1 and IGFBP-3 levels observed in individuals with infection at admission merely reflect a higher incidence of infectious complications in patients with more advanced liver cirrhosis. Actually, when evaluating the presence of infection on admission in relation to the Child-Pugh’s classification, this complication was more common in the highest stages when compared to patients classified as Child-Pugh A (data not shown).
We acknowledge some limitations to our analysis. The relatively small number of patients included may limit the extrapolation of the results. However, previous studies evaluating IGF-I and IGFBP-3 levels in patients with cirrhosis included a smaller number of subjects and were performed outside the context of acute decompensation. To our knowledge, this is the first study investigating patients admitted to the emergency department due to complications of cirrhosis. In this context, we believe that our data brings an important contribution and may represent a start point for cohort studies aimed at investigating the prognostic significance of IGF-I and IGFBP-3 in cirrhotic patients admitted for acute decompensation. Another limitation that we should highlight is the sensitivity of the test used for determining IGF-I levels. Despite the methodology being widely accepted and validated, the analytical sensitivity of the method proved to be low in the setting of liver cirrhosis, since a large proportion of the patients (36%) exhibited values below the detection limit. Consequently, the use of more sensitive techniques is advised for future studies, especially in the evaluation of prognostic significance of IGF-I in liver cirrhosis.
Based on these data, we conclude that IGF-I and IGFBP-3 serum levels were associated with variables related to the intensity of liver dysfunction and to more advanced liver disease in cirrhotic patients admitted to the hospital due to complications of the disease. The levels of these markers seem to undergo little influence from other clinical and laboratory variables, therefore mainly reflecting the hepatic functional status.
Potential Conflict of InterestNothing to report.
Financial SupportSupported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).