Recent translational research indicated a bidirectional relationship between NASH (non-alcoholic steatohepatitis) and periodontitis; however, few clinical cohorts have studied this in detail. Thus we investigated this assumed association in a well-defined cohort.
Materials and MethodsData were generated prospectively for 132 patients (32 patients with NASH and 100 unselected, consecutively collected, anonymized controls from a local dental practice): detailed periodontal parameters, i.e., pocket-probing-depths (PPD), bleeding-on-probing (BOP), plaque-index, and utilization of dental care were assessed and correlated with relevant hepatic parameters (liver stiffness via fibroscan, AST, ALT, bilirubin, and MELD-score). Gingiva samples were tested for Porphyromonas gingvalis (P.g.) and Actinobacillus actinomyctemcomitans (A.a.) by PCR.
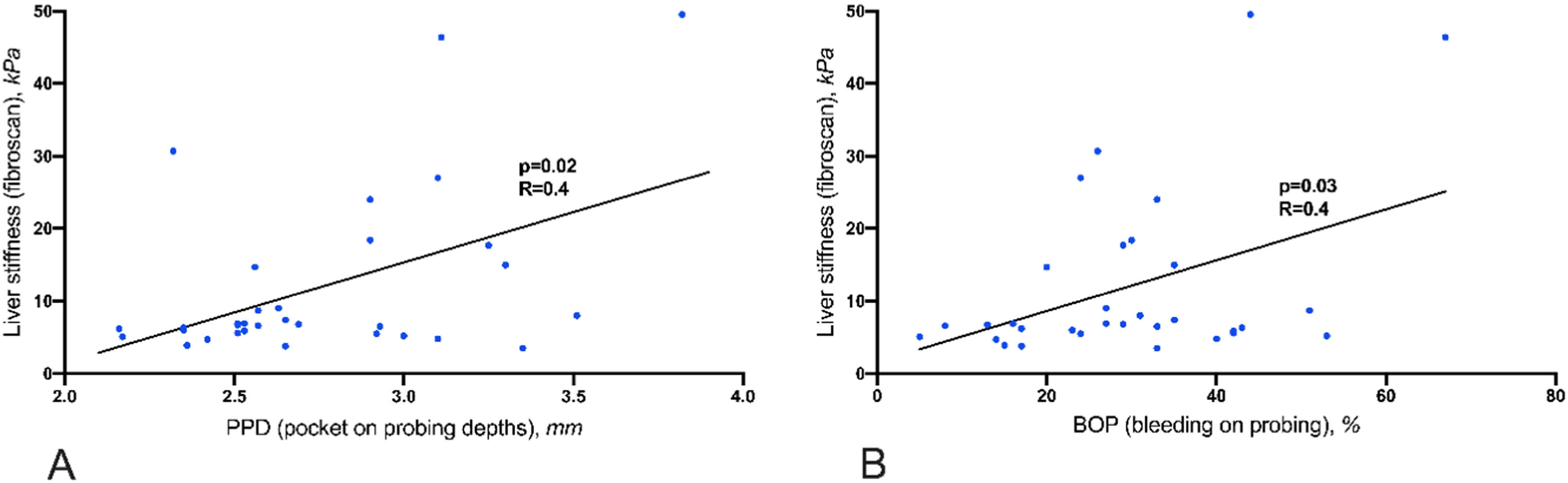
Results87.5% of NASH patients and 47% of controls suffered from moderate to severe periodontitis (p=0.01). Liver stiffness was significantly correlated with elevated PPD (p=0.02) and BOP (p=0.03). 34 % of the NASH patients did not make use of regular dental health care. In these patients, AST (p=0.04), MELD score (p<0.01), and liver stiffness (p=0.01) were significantly elevated compared to those who see a dentist regularly. The severity of NASH was not associated with the intraoral detection of P.g. and A.a.
ConclusionsThe present study suggests that NASH might be associated with periodontitis, irrespective of the intraoral presence of P.g. and A.a. Moreover, regular dental care utilization might mitigate the course of NASH, and patients should be reminded by their hepatologists of the importance of regular dental visits. Future studies should investigate the role of regular dental care and additional anti-inflammatory treatments of the oral cavity.
Non-alcoholic fatty liver disease (NAFLD) is defined as the excessive accumulation of lipid droplets within hepatocytes in the absence of excessive alcohol consumption, viral infections, or autoimmune diseases. NAFLD has a high global prevalence of approximately 25% that is expected to even increase in the future, parallel to the increment numbers of obese people with metabolic syndrome. The overarching term NAFLD is comprised of different entities: a) simple fatty liver, b) non-alcoholic fatty liver (NAFL) and c) non-alcoholic steatohepatitis (NASH), which is the histology-proven inflammatory variant of a) and b) (REF). NASH is defined as NAFL accompanied by hepatic inflammation and is associated with a more severe progressive course of the disease, leading to fibrosis or even cirrhosis [1]. Besides known risk factors for NASH, like obesity, fatty diet, dyslipidemia, and diabetes, periodontitis as a modulating risk factor for NASH has gained increased attention in recent years [2,3]. Experimental models have highlighted mechanisms connecting microbiota to the development of liver dysfunction in non-alcoholic steatohepatitis (NASH) [4]. Thus the possible therapeutic role of modification of oral microbiota for the treatment of liver diseases has been discussed [5].
In particular, it has been hypothesized, based on these models, that periodontitis may lead to systemic inflammation and increase oxidative stress and thereby contributing to the onset and progression of NASH [6–11]. However, the putative role of periodontitis in the pathogenesis of NASH and its role in the oral-gut-liver axis in real-world cohorts of NASH patients remains to be clarified [3].
Periodontitis is a chronic inflammatory disease initiated and perpetuated by an intraoral microbiological dysbiosis that supports the progressive destruction of the periodontal ligament, connective tissue, and alveolar bone and, if left untreated, results in tooth loss [12,13]. Although the majority of microorganisms colonizing the oral cavity are compatible with periodontal health, a subset of species may cause or contribute to intraoral dysbiosis and, subsequently, to the clinical signs of periodontal disease. In this regard, around 15 to 20 bacterial species are closely associated with periodontal disease.[14] Amongst them, some species, e.g., Porphyromonas gingivalis (P. gingivalis) and Aggregatibacter actinomyetemcomitans (A. actinomyetemcomitans), seem to be the most virulent.[15] While the relevance of p. gingivalis for NASH has already been studied in some pilot examinations, the knowledge regarding a possible association between A. actinommyetemcomitans and NASH is still limited.
Several animal studies have demonstrated that oral administration of periodontopathic bacteria, including the aforementioned P. gingivalis and A. actinomycetemcomitans, was associated with changes in gut microbiota, as well as in glucose and lipid metabolic pathways, leading to insulin resistance and fat deposition in the liver [16,17] Moreover, it could be demonstrated, that P. gingivalis infection of ligature-induced periodontitis increased serum levels of alanine aminotransferase as well as hepatic fat deposition in rats with high-fat diet-induced obesity and insulin resistance [9,18]. Interestingly, it could be demonstrated that the elimination of P. gingivalis infection by azithromycin inhibited NASH progression in mice receiving a high-fat diet [19], underlining the notion of P. gingivalis being involved in the pathogenesis of NASH.
Therefore, the present study aimed to analyze the frequency and degree of periodontitis and the presence of P. gingivalis and A. actinomycetemcomitans in a well-characterized, prospectively recruited cohort of NASH patients and its association with disease activity.
2Material and MethodsIn this prospective study, adult NASH patients of the liver outpatient clinic of the University Hospital Hamburg-Eppendorf between 03/2021 and 07/2021 have been asked to participate. Thirty-two of them gave written informed consent and were included in this study. NASH has been diagnosed previously based on clinical criteria, i.e., systemic levels of liver enzymes in association with liver elastography results and biopsies.
Intraoral examination (full mouth charting) was done by a calibrated dentist (A.S.). The intraoral examination included dental status (number of teeth and number of teeth with cavities), mucosal status, mouth hygiene (sulcus bleeding index), and probing of pocket depths at six locations, as usual in dental medicine. Periodontal disease has been graded according to the EFP/ORCA guidelines/recommendations [20].
All patients underwent a standardized interview and responded to a questionnaire regarding the frequency of their dentist visits and smoking behavior, among other parameters.
P. gingivalis and A. actinomycetemcomitans were identified by species-specific PCR as described previously [21]. A probe was inserted into the deepest gum pocket of a sextant and wiped on a cotton swab, which was transferred went to PCR. Laboratory data and baseline patient characteristics, such as ALT, AST, bilirubin, age, clinical attachment loss (CAL, i.e., the most important parameter to assess periodontal tissue loss due to periodontal disease), smoking status, and frequency of dental visits were recorded. CAL was presented as a range of CAL values in mm for each patient and as average pocket depth. To assess the status of the gingiva and the presence of periodontitis in an unselected cohort, 100 retrospectively analyzed unselected patients from a standard dental practice served as controls. These cases present the most recent 100 patients undergoing standardized periodontitis screening at this practice. Basic data like periodontal status, age and sex were gathered retrospectively for these anonymized patients in line with our local regulations and the rules of our ethical court.
Statistical analysis was performed as follows: Continuous variables with a non-normal distribution were expressed as the median and interquartile range (IQR). Groups were compared using the Mann–Whitney U-test. Categorical variables were expressed as numbers (%) and compared with Fisher's exact test. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA).
2.1Ethical statementThis prospective study was reviewed and approved by the Ethics Committee of the Medical Council of Hamburg (PV-4081 and MC-368/18). The study was performed according to the recommendations of the Declaration of Helsinki. The retrospective analysis of the control cohort was completely anonymized and therefore did not require any clarification or formal ethics committee approval according to local laws and regulations.
3ResultsA cohort of 32 well-defined NASH patients has been prospectively recruited for the present study. Sixteen patients were male (50%). The age ranged from 21 to 83 years (mean 53 years, Std. deviation 13.2). Patient characteristics are depicted in Table 1. In this cohort, only six patients (19%) were smokers; four of them were smoking more than 10 cigarettes per day, and two were less than 10 per day. Fifteen patients (47%) suffered from diabetes with HbA1c values between 5.1% and 8.0% median 6.4%).
Characteristics of patients with or without periodontitis.
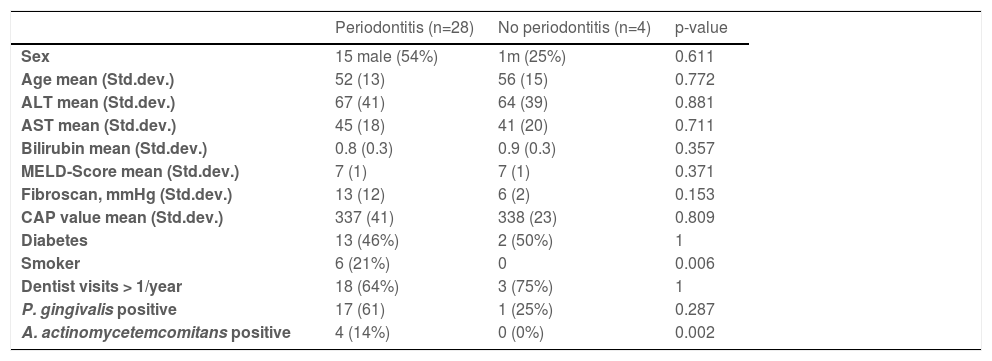
In 28/32 (87.5%) of NASH patients, periodontitis could be diagnosed: 7 of these showed slight periodontal disease (stage 2), 14 moderate periodontal disease (stage 3) and seven advanced periodontal diseases (stage 4). Only four patients presented with gingivitis without loss of bone (stage 0). No patient presented with stage 1. The maximal periodontal pocket depths ranged from 3mm to 9mm (median 6mm). None of these patients received specific treatment for periodontitis by a dentist within the last two years, but 13/32 (41%) knew they had periodontitis. Detailed patient characteristics of patients with and without periodontal disease are shown in Table 1.
According to the examination of the patients by an experienced dentist (A. Shiprov), oral hygiene has been classified into good hygiene (11/32, 34%), moderate hygiene (4/32, 13%), reduced hygiene (7/32, 22%) and bad hygiene (10/32, 31%).
11/32 patients had a complete set of teeth of, 28 regular teeth, 15 patients (47%) had lost less than 10 teeth and six patients had lost 10 or more teeth.
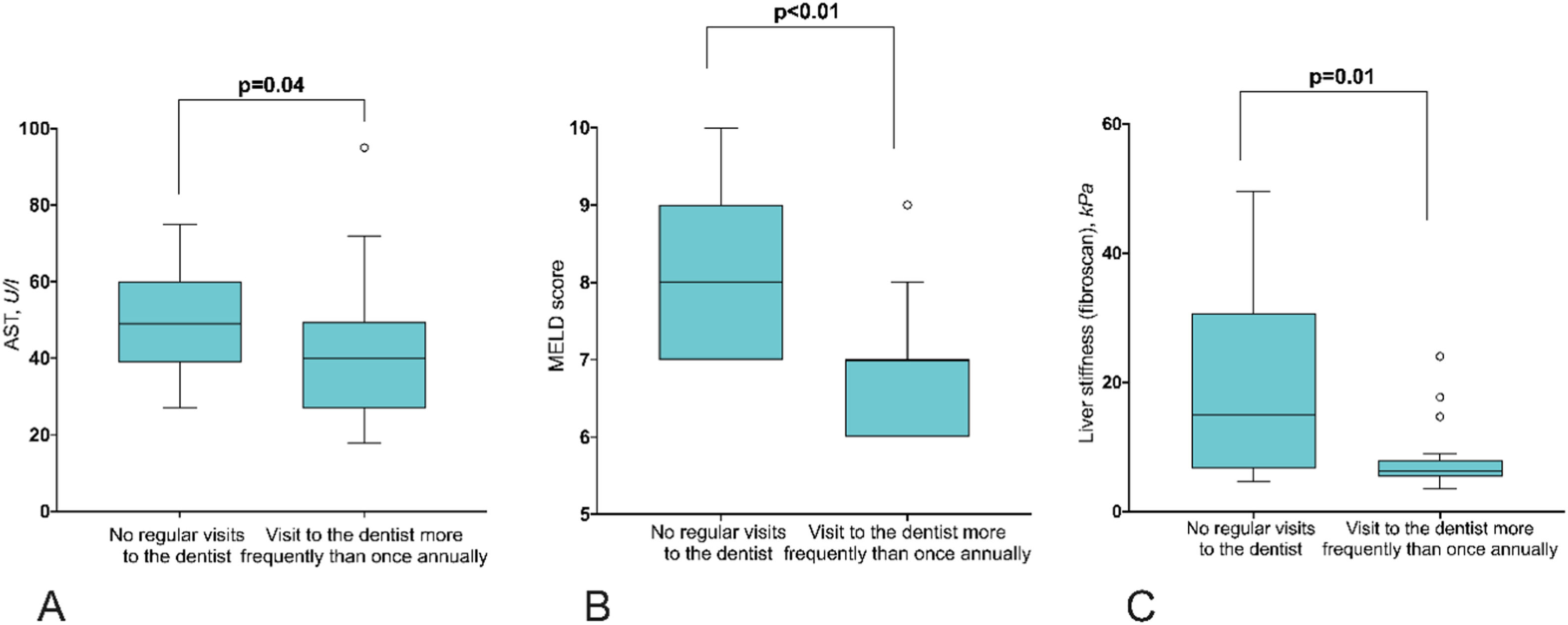
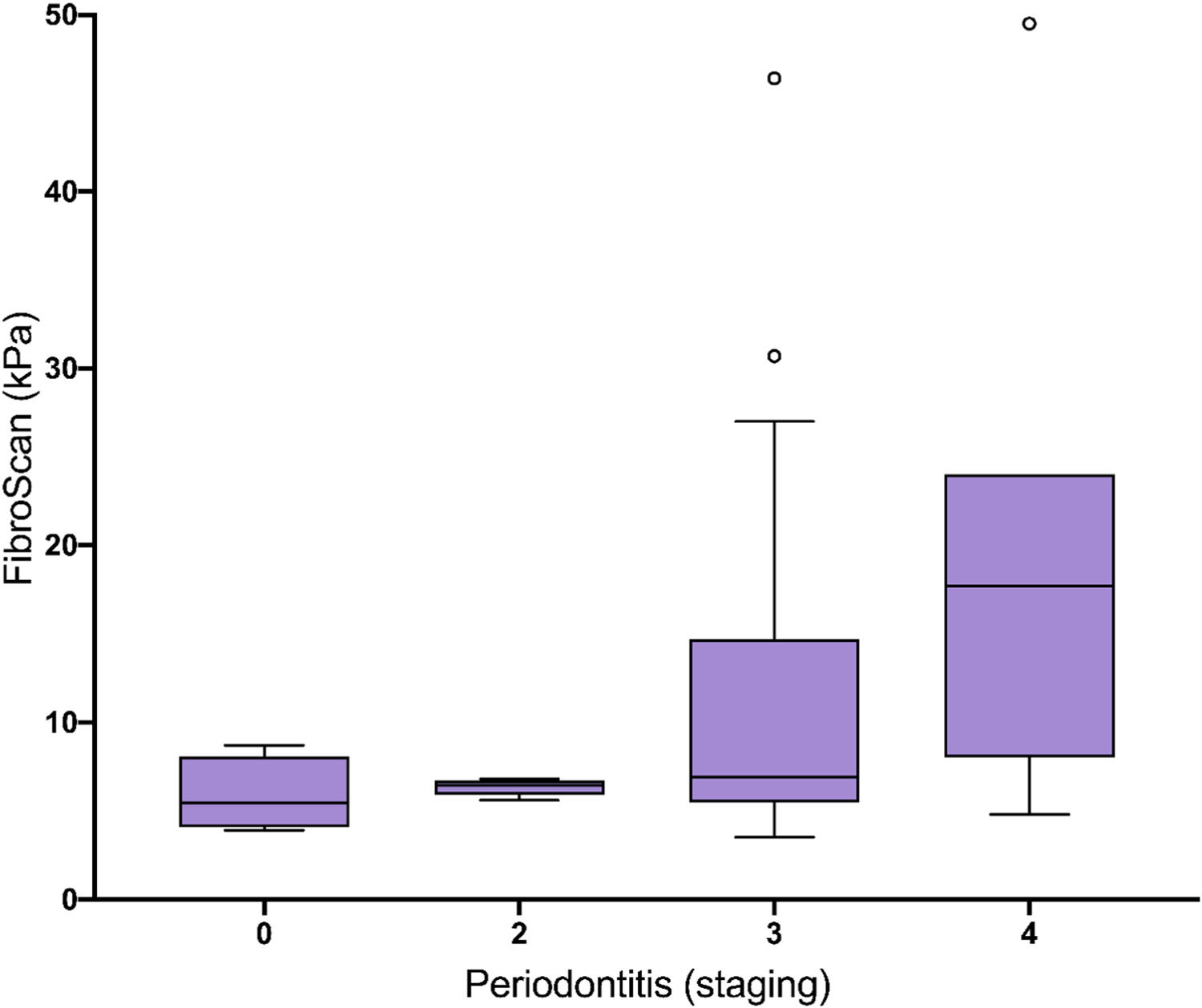
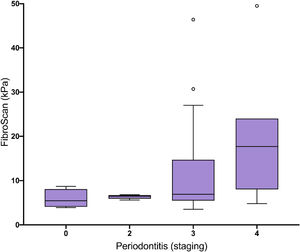
Eleven patients (34%) stated that they did not visit a dentist regularly. 11 patients (34%) visited a dentist once a year, and 10 patients (32%) had dental check-ups more than once annually. AST, MELD score and liver stiffness are significantly elevated in patients without annual regular dentist visits in comparison to patients visiting a dentist more frequently than once a year (Fig. 1). Liver stiffness values (mmHg, fibroscan) were increased with the severity of periodontitis (Fig. 2). Both classical periodontitis values, BOP (bleeding on probing) and PPD (probing pocket depth), were significantly correlated with liver stiffness (fibroscan) (Fig. 3).
The number of missing teeth was correlated with the age of the patients (R=0.447, p=0.010) and fibroscan results (R=0.374, p=0.035), but it was not correlated with ALT (R=-0.099, p=0.591), AST (R=0.150, p=0.412), bilirubin (R=-0.124, p=0.498), MELD (R=-0.013, p=0.942) or CAP value (R=0.218, p=0.238).
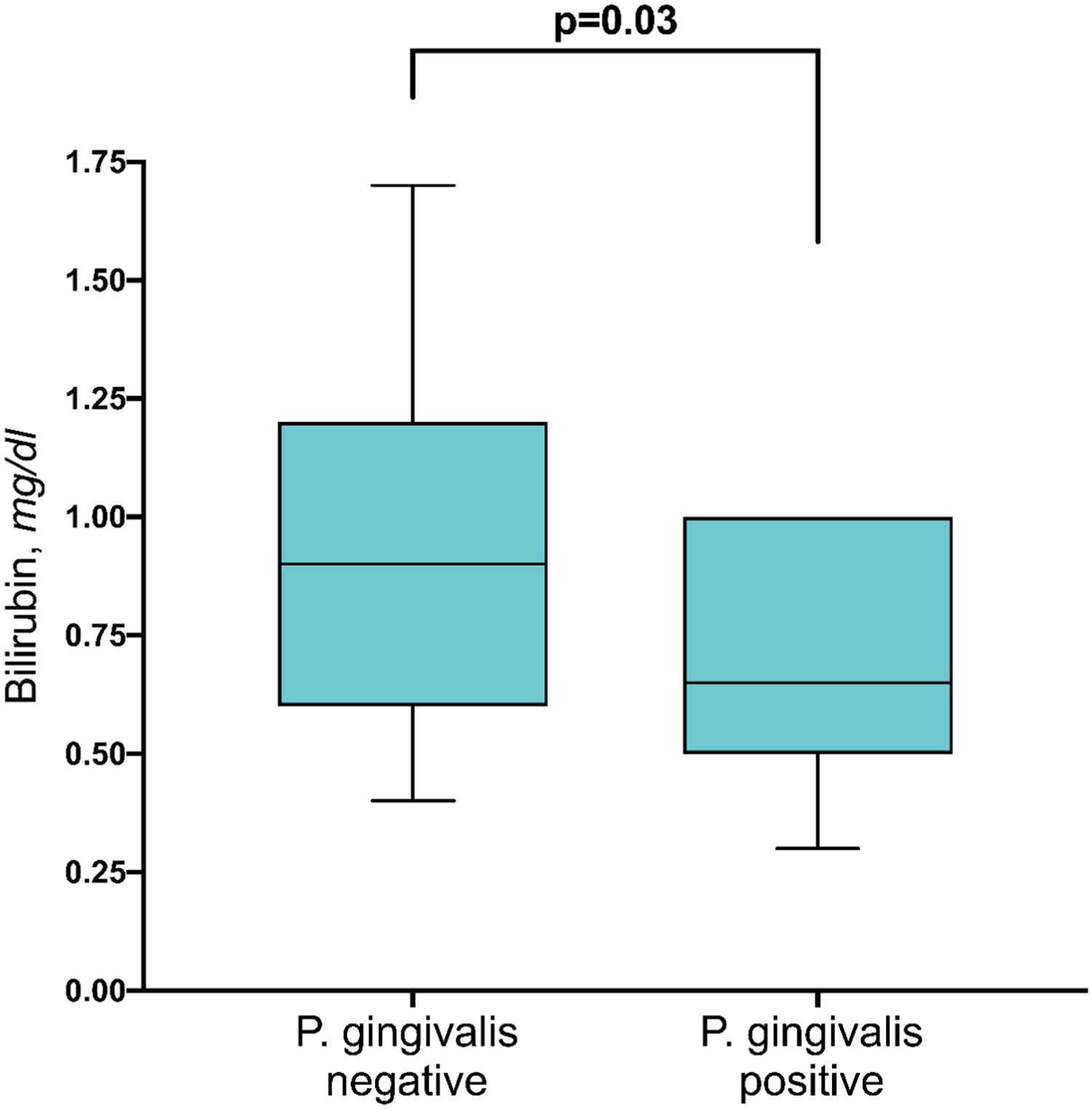
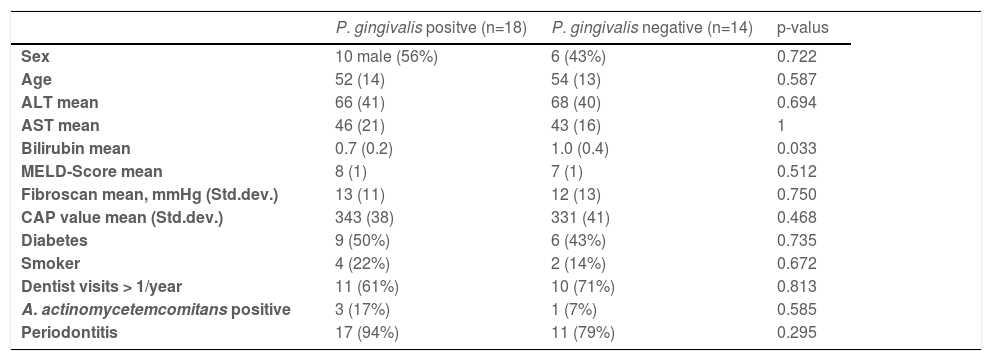
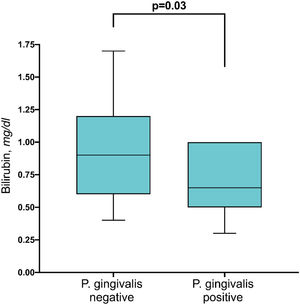
In 18 patients (56%), P. gingivalis could be detected in gingiva samples by PCR (Table 2). In 5 of these, the P. gingivalis subtype could be determined: 3 of those had the yrrB_1 variant mono, 1 had the yrrB_1 in rag A variants and 1 had rag A and B variants. The presence of P. gingivalis was not associated with age, ALT, AST, MELD-score, liver stiffness, CAP-value, stage of periodontal disease, or the number of teeth. However, serum bilirubin levels were slightly higher in P. gingivalis negative patients in comparison to positive patients (Fig. 4) (p=0.03).
Characteristics of patients with or without P. gingivalis.
In four patients (12.5%), the PCR of the gingiva sample tested positive for A. actinomycetemcomitans. The presence of A. actinomycetemcomitans was not associated with any of these factors: age, ALT, AST, bilirubin, MELD-score, liver stiffness, CAP-value, stage of periodontal disease, or the number of teeth (p=n.s.).
To compare the incidence of periodontitis in the NASH cohort with the basic incidence in the general population, a retrospective cohort (n=100) of patients from a Hamburg dental practice was analyzed. Forty-seven of these subjects were male (47%), with ages ranging from 17 to 89 years (median 51 years). Fifty-nine patients (59%) had periodontitis, which was significantly less than in the NASH cohort, with an incidence of 87.5% (p=0.01).
4DiscussionNumerous studies previously suspected an association between NASH and periodontitis in general or the presence of P. gingivalis in particular. Based on those animal experiments and observations in humans, it has been hypothesized that periodontitis may lead to systemic inflammation and increase oxidative stress and thereby contribute to the onset and progression of NASH [6–11]. Increased levels of endotoxin derived from P. gingivalis infection appear to play a considerable role in the progression of NASH by a complex cytokine cascade.
However, this small pilot study investigates whether there is a real-world association between the presence of periodontitis, P. gingivalis, and A. actinomycetemcomitans and the severity of liver damage in a well-defined cohort of NASH patients. In line with other studies, our results identified an association between periodontitis and smoking or carriership of A. actinomycetemcomitans (Table 1).
The current pilot study indicates that the majority of NASH patients suffer from periodontitis. Thus, the experimentally assumed association between NASH and periodontitis is highly probable. It is astonishing that the treatment of periodontitis in NASH patients has not been actively studied to mitigate immune activation, the microbiome, and NASH activity.
Interestingly, it was shown in the current study that 34% (11/32) of the NASH cohort visited a dentist less frequently than once per year. In 4/11 (36%) of these, severe periodontitis above stage 4 could be detected. Thus, one recommendation resulting from this study is that NASH patients should be reminded by their hepatologists of the importance of regular dental visits.
Furthermore, AST, MELD, and liver stiffness values were found to be significantly worse in patients who visit the dentist less frequently than once a year than in patients who visit the dentist regularly (Fig. 1). In Germany, six-monthly visits to the dentist are recommended. Thus, it appears that patients who do not follow this recommendation have a worse liver status based on AST, MELD, and liver stiffness. This illustrates that reduced health awareness in general or other factors (e.g., psychological or sociological) may likewise be associated with bad dental care and a more severe course of NASH. The relevance of this observation becomes clear when one considers that a large European study investigating 27334 individuals revealed that the Gross Domestic Product was positively associated with sugar consumption in European countries and that lower education groups had poorer diets [22].
As an alternative explanation, one could imagine that untreated periodontitis worsens the course of NASH. This possible explanation is in line with a recently published report, investigating the association between NAFLD and periodontal disease in a cross-sectional study [23]. In this important study on 164 NAFLD patients, P. gingivalis positivity correlated with liver stiffness determined using magnetic resonance elastography. However, the current study does not allow us to determine with certainty whether there is a causal relationship between periodontitis and NASH or whether the common variable linking the presence of periodontitis and NASH is reduced health awareness in these patients. None of the studied patients received periodontitis-specific treatment within two years, but 13/32 (41%) of them did know that they were suffering from periodontitis. This demonstrates the reduced health awareness and reduced care for their bodies in these patients. However, the possibility that dental treatment of your periodontitis might improve the inflammation needs to be tested. Recently a randomized controlled 2-arm study investigated 40 patients with NALD and periodontitis [24]. Stratified by age and sex, a group of 20 patients was randomized to either a group receiving scaling and root planning treatment or a tooth-brushing group. Transaminase levels and P. gingivalis IgG antibodies significantly stronger decreased in the group receiving scaling and root planning treatment in comparison to the tooth-brushing group. Thus periodontal treatment may better the liver status in NASH patients. Further studies are needed to validate these findings.
The limitations of this study are clear: on the one hand, the cohort in this pilot project is small and in a larger cohort further variables could be controlled for; on the other hand, the observations are unicentric and it is uncertain whether they can be generalized to the whole of Germany or even worldwide.
Further, larger studies with standardized psychological questionnaires, as well as prospective therapeutic studies, are needed to clarify a possible link between NASH and periodontitis is causative or if it is founded on a common factor, like behavior.
Last but not least, a completely novel and surprising result came up in the current study: P. gingivalis positive patients had significantly lower bilirubin values in comparison to negative patients (Fig. 4). The relevance of this finding is still unknown. As mean bilirubin levels were not increased, we do not overestimate this finding. Future larger prospective studies controlled for further variables will show if this minor finding in this small pilot study will be confirmed.
5ConclusionsThe current pilot study suggests that NASH might be associated with periodontitis, irrespective of the intraoral presence of P.gingivalis and A. actinomyetemcomitans. Moreover, regular dental care utilization might mitigate the course of NASH, and patients should be reminded by their hepatologists of the importance of regular dental visits. Future studies should investigate the role of regular dental care and additional anti-inflammatory treatments of the oral cavity.
Author contributionsSP, the conceptualization of the study, writing of the manuscript, responsible for the integrity of the work; AS, studying patients, writing of the manuscript; UP, PCR testing, writing of the manuscript; JSzW, patient care, writing of the manuscript; JK, patient care, writing of the manuscript; FF, PCR testing, writing of the manuscript; MM, coordination of samples, patient care, writing of the manuscript; TF, patient care, writing of the manuscript; KH, patient care, writing of the manuscript; TH, care, writing of the manuscript; GA, patient care, writing of the manuscript, the conceptualization of the study; TB, writing of the manuscript, the conceptualization of the study.
Data availability statementThe authors confirm that the data supporting the findings of this study are available within the article.