The present study was conducted to investigate effect of pentoxifylline (PTX) on acute liver injury caused by galactosamine (D-Gal) in rats and the underlying mechanism involved in this setting. Moreover, we attempted to compare its effect to the well-established hepatoprotective agent, silymarin (SYM). The rats were randomly assigned 5 groups, control, PTX-treated (100 mg/kg, 3 weeks), SYM-treated (100 mg/kg, 3 weeks) and their combination. Hepatic injury was induced by intraperitoneal single dose injection of D-Gal (800 mg/kg). Hepatic functions parameters, including serum albumin and alkaline phosphatase (ALP) levels were determined. Antioxidants enzyme activities such as superoxide dismutase (SOD), catalase (CAT) as well as lipid peroxides and hepatic total nitrites were measured. Besides, histopathological examination was also performed using portions of liver tissues. Results showed that the liver injury induced by D-Gal was improved in the three pretreated groups to variable extents. Pretreatment with PTX prevented D-Gal-induced reduction of antioxidante enzyme activities, SOD and CAT, and attenuated the elevated malonaldahyde (MDA) level in hepatic tissue as marker of lipid peroxidation. In addition, pretreatment with PTX resulted in an increase in hepatic triglycerides, normalization of nitric oxide level, and lowering serum ALP activity as well as inhibited the decreased serum albumin level caused by D-Gal. These biochemical changes were reflected on attenuation the structural alterations of the liver integrity. Collectively, our data suggest that PTX exhibits a potential hepatoprotective effect against D-Gal-induced hepatotoxicity and this effect might be attributed to its antioxidant properties.
Liver injury can be caused by different agents, such as viruses, chemicals, alcohol, and auto-immune diseases1 D-galactosamine (D-Gal) is a well established hepatotoxicant, it induces a diffuse type of liver injury closely resembling human viral hepatitis2 and acute self-limiting hepatitis with necrosis, inflammation and regeneration, resembling a drug-induced disease in humans.3 The toxicity of D-Gal is mainly related to the depletion of uridine pools that are associated with limited ribonucleic acid (RNA) and protein synthesis, thus altering hepatocellular function.4
Different pharmacological compounds have been tested to reduce acute or chronic inflammatory responses, which may damage liver function. It has been demonstrated that phosphodiesterase inhibitors may be protective in various liver injury models.5,6 Pentoxifylline (PTX) (non-selective phosphodiesterase inhibitor) is a methyl xanthine derivative that has been used for its regulatory effect on blood flow. PTX increases the flexibility of red and white blood cells, reduces the blood viscosity by decreasing plasma fibrinogen concentrations and decrease platelet aggregation and thrombus formation. Polymorphonuclear leukocyte-mediated effects, such as superoxide production, chemotaxis, phagocytosis and tumor necrosis factor (TNF) production are inhibited by PTX.7
Silymarin (SYM) from milk thistle is a hepatoprotective drug was widely used in the world. SYM may reduce free radical production and lipid peroxidation in the setting of hepatotoxicity as it has powerful antioxidant properties.8 SYM may act through its ability for toxin blockade; it may bind to the hepatocyte cell membrane receptor site, inhibiting binding of toxins to these sites.
The aim of the present study was to examine the putative hepatoprotective effects of PTX against hepatotoxicity induced by D-Gal and elucidate the underlying mechanisms involved in this protection, as a sole effect or synergistic with SYM.
Material and MethodsAnimalsAnimals used in the present study were male albino rats (8 weeks of age) weighing 180-200 g. Animals were kept under constant environmental conditions throughout the period of the experiment. Animals were exposed to dark/light cycle of 12:12 hours. Experiments were conducted in accordance with the guidelines for animal care of the United States Naval Medical Research Centre, Unit No. 3, Abbaseya, Cairo, Egypt, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care international (AAALAC international).
Experimental proceduresAll animals were fed standard animal chow along the period of the experiment (21 days). After the acclimatization period, the rats were divided into five groups, each of 12 rats. Firstly, control group; in which animals received saline 0.5 mL/day i.p. for 3 weeks. Second, D-GAL-treated group; in which animals received saline 0.5 mL/day i.p. for 3 weeks and at 21st day received a single dose of D-GAL. Third, PTX-treated group; animals received PTX (100 mg/kg/day, i.p.) dissolved in 0.5 mL saline for 3 weeks. Fourth, SYM-treated group; in which animals received SYM (100 mg/kg/day, oral) suspended in 0.5 mL 0.1% starch solution for 3 weeks. Fifth, PTX + SYL group; animals received PTX followed by simultaneous administration of SYM for 3 weeks by the same previous doses as previously described.10
All groups except group 1 received at 21st day a single dose of D-Gal (800 mg/kg, i.p.) once in the morning. At the end of the study, the animals were sacrificed and blood was collected for separation of sera. The livers were isolated, washed off blood and connective tissue was removed then portions of liver tissues were separated and kept in 10% formalin for histopathological examination.
Immediately, the rest of liver tissues were immediately flash-frozen in liquid nitrogen and stored at - 80 °C. Kits used for determination of malonaldahyde (MDA) levels (Biodiagnostic, Egypt) as a marker of lipid peroxides,11 tissue nitrite as an indicator of nitric oxide (Biodiagnostic, Egypt), albumin (DP International, Egypt), alkaline phosphatase (ALP), and total protein concentrations (Spectrum Diagnostics, Egypt), superoxide dismutase (SOD) and catalase (CAT) enzymes12 (Biodiagnostic, Egypt). In addition, serum albumin levels and ALP activities were measured in sera of all the experimental animals.
Histopathological examinationSmall pieces of the livers fixed in 10% buffered neutral formalin were processed for embedding in paraffin. Sections of 5-6 mm thickness were stained with hematoxylin and eosin, examined for histopathological changes.
Statistical analysisResults were expressed as means ± standard error of the mean (SEM) and were analyzed for statistically significant difference using one-way analysis of variance (ANOVA) followed by the Tukey-Kramar post analysis test. P values less than 0.05 were considered significant.
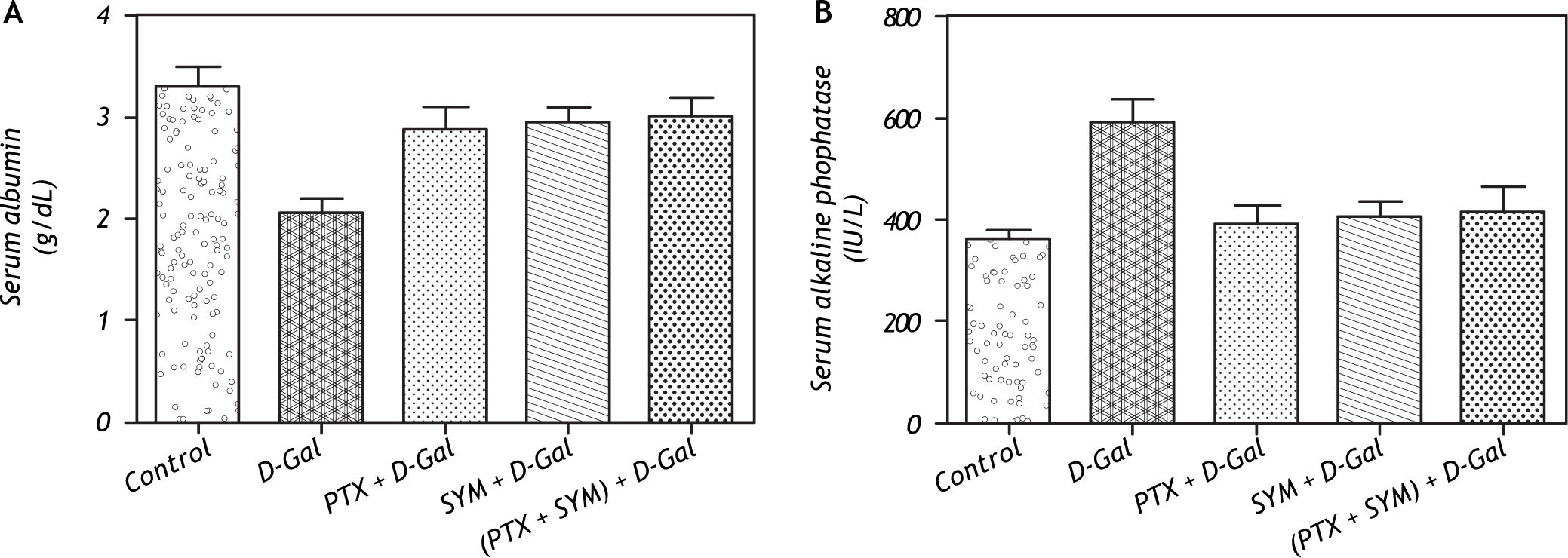
ResultsEffects of PTX on the serum albumin and ALP levelsD-Gal exhibited a significant reduction of serum albumin level when compared to control rats. However, the individual or combined administration of either PTX or SYM resulted in a significant elevation in serum albumin level nearly to the control level (Figure 1A). Administration of D-Gal resulted in a significant elevation of serum ALP activity in comparison to the control rats. Meanwhile, the single or combined administration of either PTX or SYM exerted a marked reduction in the serum ALP activity to the normal level (Figure 1B).
Effect of D-Gal on the serum albumin and alkaline phosphatase levels in normal rats and rats pretreated with PTX, SYM and their combination. A. Effect of D-Gal on the serum albumin level in normal rats and rats pretreated with PTX, SYM and their combination. B. Effect of D-Gal on the serum alkaline phosphatase in normal rats pretreated with either PTX or SYM or their concurrent administration. Values of each bar represent the mean ± SEM of 7 observations. *Statistically significant of D-Gal-treated group from control group at the significance level p < 0.05. #, f, δ Statistically significant of PTX, SYM and (PTX and SYM) versus D-Gal-treated group, respectively, at the significance level p < 0.05. D-Gal: D-Galactosamine. PTX: Pentoxifylline. SYM: Silymarin.
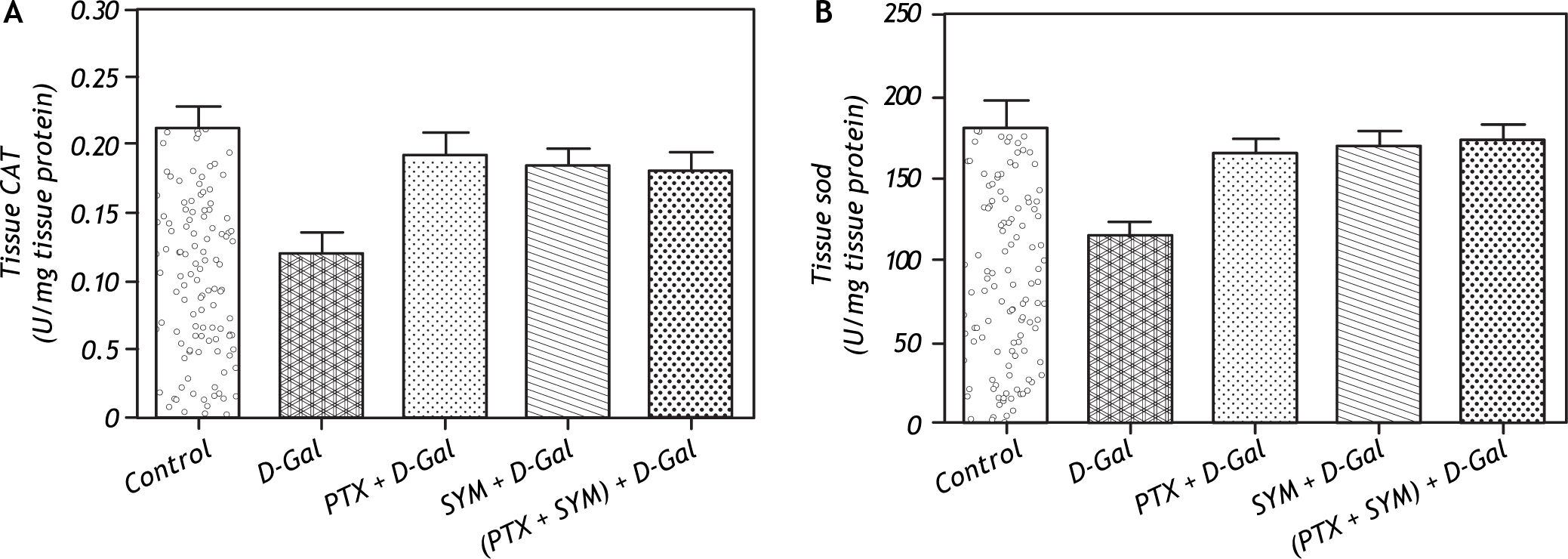
D-Gal produced a significant decrease of CAT activity when compared to control rats. Pretreatment with either PTX or SYM significantly increased CAT activity of liver tissue compared to D-Gal-treated group. Combined administration of PTX and SYM significantly increased the hepatic CAT activity compared to D-Gal-treated group (Figure 2A). The activity of SOD was significantly reduced in D-Gal group as compared to control rats. In PTX pretreated group, a significant increase of SOD activity was observed compared to D-Gal-treated group. Meanwhile, pretreatment with SYM or (PTX + SYM) significantly increased SOD activity when compared to D-Gal-treated group (Figure 2B).
Effect of D-Gal on antioxidant enzyme activates of liver tissue in normal rats and those pretreated with PTX, SYM and their combination. A. Effect of D-Gal on CAT of liver tissue in normal rats and those pretreated with either PTX or SYM or their combination. B. Effect of D-Gal on CAT of liver tissue in normal rats pretreated with PTX, SYM individually or in combined administration. Values of each bar represent the mean ± SEM of 6 observations. * Statistically significant of D-Gal-treated group from the control group at the significance level p < 0.05. #, f, δ Statistically significant of PTX, SYM and (PTX and SYM) versus D-Gal-treated group, respectively, at the significance level p < 0.05. CAT: Catalase. SOD: Superoxide dismutase and other symbols as in Figure 1.
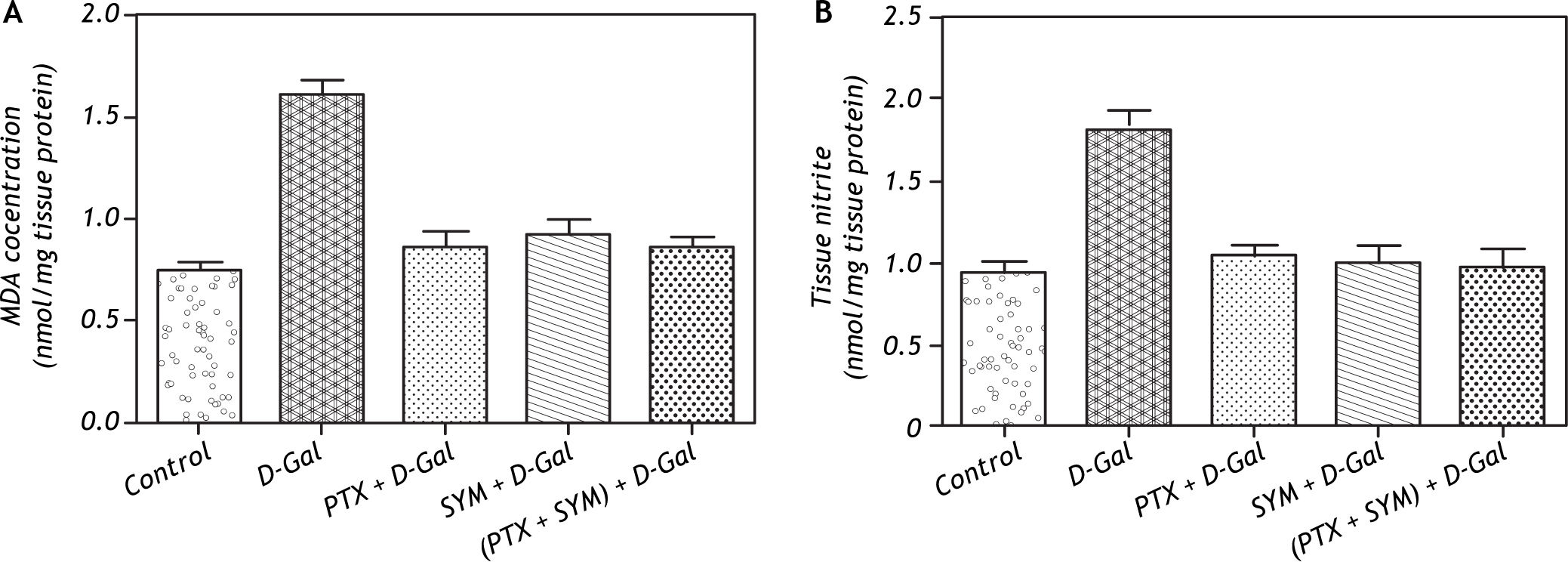
Intraperitoneal administration of D-Gal significantly elevated the hepatic tissue MDA concentration (a biochemical marker of lipid peroxidation) to about two-fold of the value observed in control group (Figure 3A).
Effect of D-Gal on hepatic lipid peroxidation and total nitrites in normal rats and those pretreated with PTX, SYM and their combination. A. Effect of D-Gal on the hepatic lipid peroxidation in normal rats pretreated with either PTX or SYM or their combination. B. Effect of D-Gal on the hepatic total nitrites in normal rats pretreated with PTX and SYM individually or in concurrent administration. Values of each bar represent the mean ± SEM of 7 observations. * Statistically significant from the control group at the significance level p < 0.05. #, f, δ Statistically significant of PTX, SYM and (PTX and SYM) versus D-Gal-treated group, respectively, at the significance level p < 0.05. MDA: Malondialdhyde and other symbols as in Figure 1.
On the other hand, the total hepatic nitrite level was significantly elevated in D-Gal treated groups compared to the control group. However, the single or combined administration of PTX and SYM significantly restored the elevated hepatic total nitrites to the normal level (Figure 3B).
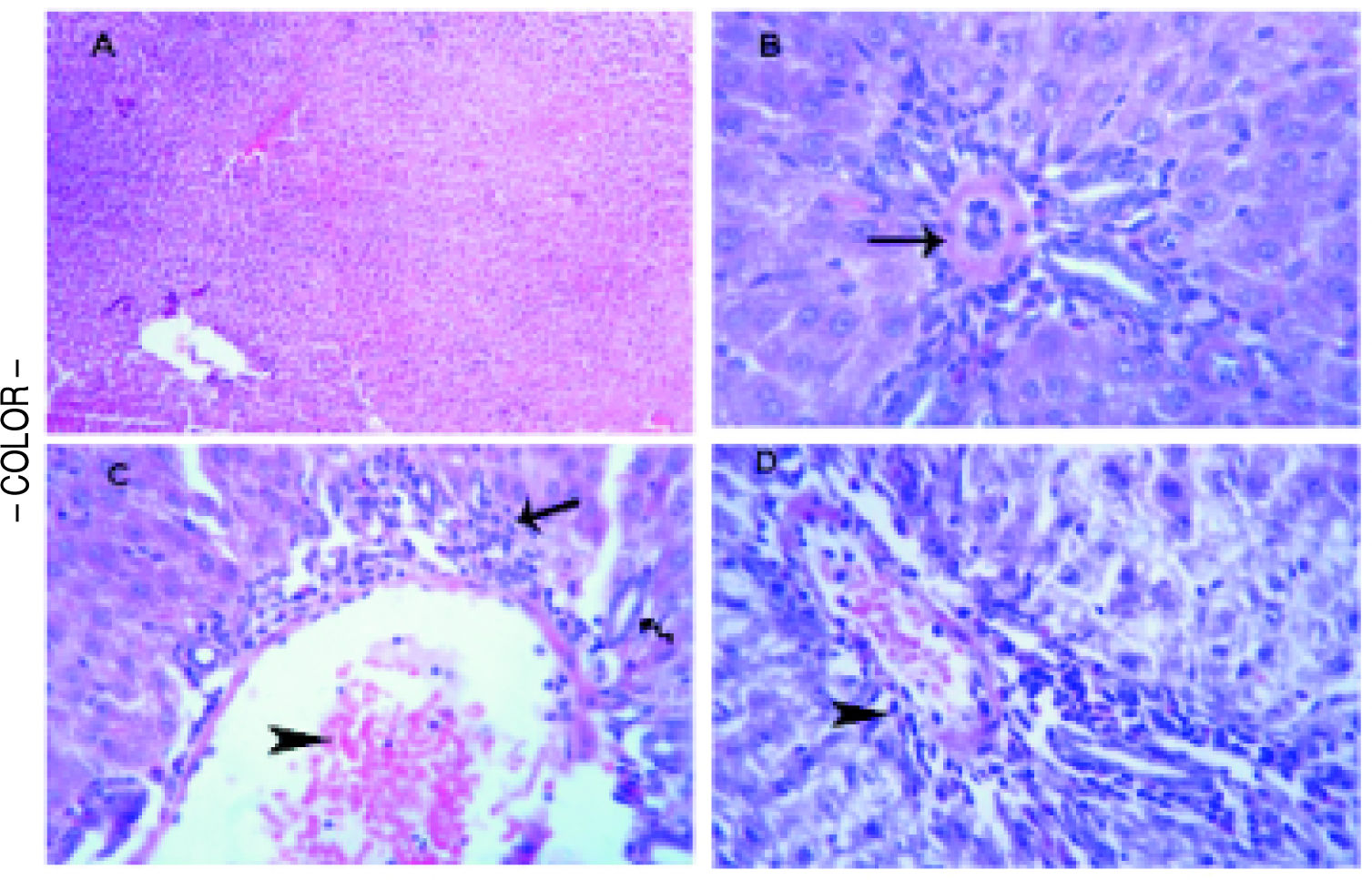
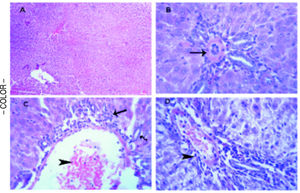
Histopathological examinationTo verify the biochemical changes in liver, the histopathological examination of liver both of the control and treated rats was performed. The liver of the control group was normal with no evidence of any microscopic abnormalities (Figure 4A). In D-Gal-treated group, microscopically, vasculitis, represented by congested blood vessels and perivascular edema and few lymphocytes infiltrations was noticed. Thickening and hyalinization of the tunica media of hepatic arterioles were observed (Figure 4B). In PTX group, microscopically, some portal areas showed congested blood vessels and leukocyte margination and emigration besides mild hyperplasia of the epithelial lining of the bile ducts and few round cell infiltrations (Figure 4C). In SYM-treated group, few round cells (mainly of lymphocytes) in the portal areas and the interstitial tissue were detected (Figure 4D).
Histopathological analysis to the effects D-Gal on liver in the absence or present of either PTX or SYM. (A) Liver of the control group illustrates normal parenchymal and sinusoidal structures, H&E. (B) Liver of D-Gal-treated group showing portal area with thickening and hyalinization of the tunica media of hepatic arteriole (arrow), H&E. x 500. (C) Liver PTX-treated group reveals portal area with congested blood vessel (arrow head) and few round cell infiltrations (arrow), HE x 300. (D) liver of SYM-treated group shows portal area with few round cell infiltrations (arrow head), HE x 300.
The current study investigated the protective effects of PTX on experimentally induced hepatic toxicity. Hepatic injury in the study was induced by using a single dose of D-Gal, which is a hepatotoxicant, and inducer in hepatic injury models, both in vivo and in vitro. In vivo, D-Gal has been reported to cause hepatic damage resembling viral hepatitis and drug induced hepatitis.13
SYM was used in the present study as a reference and a well-established hepatoprotective agent. This compound has been demonstrated to exhibit hepatoprotectve properties in both in vitro14 and in vivo15 studies. SYM proved to be protective against several hepatotoxicants such as carbon tetrachloride,16 paracetamol,17,18 and D-Gal18 in different animal models.
The current study showed that D-Gal caused a significant decrease in serum albumin level as an indicator of hepatotoxicity, supporting the previous study of Wong, et al.19 Moreover, it is has been shown that the lowering of serum albumin level is attributed to the reduction of albumin mRNA expression, this report observed that there was an abrupt decrease of albumin mRNA which was maximum at the day two after D-Gal administration.20 Noteworthy, the present data showed that pretreatment with PTX significantly inhibited the reduction in serum albumin in D-Gal treated rats compared to control rats. This effect could be explained by ability of PTX to increase the blood flow and irrigation to liver and this might contribute in returning liver vitality. The same effect of PTX on serum albumin level was previously reported.21
On the other hand, D-Gal caused an increase in serum ALP compared to non-treated control group. This rise in serum ALP activity may be attributed to the disturbance in the secretory activity or in the transport of metabolites or may be due to altered synthesis of certain enzymes as in other hepatotoxic conditions.22 The present finding is in accordance with the result of a previous report of Abdel Salam, et al.23 demonstrating an increase of ALP activity in carbon tetrachloride and acetaminophen models of liver injury Serum ALP activity in PTX pretreated group was returned to approximately its normal level compared to D-Gal treated group. This effect, also, was comparable to the effect of SYM on ALP activity in serum, in accordance to the study reported by Peterson.21 We suggest that the bile duct-obstruction may cause more pronounced elevation in ALP activity as ALP is mainly produced in bile duct and its release is enhanced by cholestasis. Thus, PTX failed in preventing that effect.
In the current work, administration of single dose of D-Gal caused non significant decrease in triglycerides concentration in the liver, this effect may be due to impairment of hepatic synthesis of triglycerides. Kattermann and Sirowej demonstrated that such decrease could be attributed to sex differences in the fatty liver induced by D-Gal, and the level of hepatic triglycerides content decreased especially in male animals rather than females.
Oxidative stress and lipid peroxidation that are mediated by oxygen free radicals has been implicated as a common link between chronic liver damage and hepatic fibrosis.25 Hepatocytes are well recognized as being continuously exposed to reactive oxygen species in various liver diseases including cholestasis. Antioxidant molecules such as glutathione (GSH) and antioxidative enzymes such SOD, and CAT ordinarily provide hepatocytes with resistance to oxidative stresses.26,27 The reduction of oxidative stress by PTX might be important because increased oxidative stress is a feature of the CCl4-induced liver injury and significant protection was obtained with the use of antioxidants.28
Notably, our study revealed that the single intraperitoneal administration of D-Gal resulted in a significant reduction of tissue catalase and superoxide dismutase activity in addition to elevated level of thiobarbituric acid reactive species (TBARs) as a biochemical marker of lipid peroxidation. It has been suggested that oxygen derived free radicals released from activated hepatic macrophages are the primary cause of D-Gal induced liver damage.29,30 However, Wong, et al.19 reported an increase in SOD activity which contradicts the present results. They speculated that the upregulation of SOD activity may be attributed to adaptive mechanisms to counteract increased oxidative stress.
There are several studies reporting the relevance of CAT to hepatic fibrosis i.e. CAT blocked Tumor Growth Factor B1 (TGF-B1) and collagen production in hepatic stellate cells.31 CAT activity was reduced in human hepatic injury and cirrhosis32 and overexpression of CAT rescued hepatic damage33. A possible explanation of the effect of D-Gal on CAT activity is the assumption that the impairment of CAT activity might be the cause rather than a consequence of D-Gal hepatotoxicity. Thus, the increase in oxidative stress caused by impaired CAT activity is likely a key element of triggering hepatic fibrosis. This is in agreement with the reported effect of thioacetamide (another hepatotoxic agent) on catalase activity.34 The reduction of CAT activity observed in D-Gal treated group in the study was, also, reported in pervious studies.35-37 Pretreatment with PTX prevented D-Gal induced reduction of antioxidative enzymes, SOD and CAT and attenuated the elevated MDA level in hepatic tissue, which observed in control group. This effect may be due to increased blood flow to liver which increases its antioxidant capacity in addition of direct antioxidant effect of PTX and its metabolites. As SYM has strong antioxidative properties and is able to scavenge both free radicals and reactive oxygen species, so it exhibited the same results observed with PTX pretreated group.
There is good evidence that cyclic nucleotides are able to prevent oxidative stress by reduction of lipid peroxidation.38 Demir and Inal-Erden39 explained the effects of PTX on production of free radicals to be linked with leukocyte-driven radicals. PTX can enhance the chemotactic response of neutrophils. PTX, also, may inhibit the phagocytosis and superoxide production by neutrophils and monocytes. Savas, et al.40 reported that PTX reduced levels of MDA as well as SOD and CAT activities in a rabbit model of ischemia-reperfusion injury, they attributed these effects to their assumption that PTX is a free radical scavenger and neutrophils stabilizer. Thus, PTX decreases free radical production, antioxidant enzymes and cytokines released from the activated neutrophils. Collectively, these reports support the hypothsis that PTX might exert its hepatotprotective effects due its antioxidant properties.
Nitric oxide plays a role in pro-inflammatory cell signaling, altering cellular gene expression, enzyme activity and transcription factor activity. In addition, NO regulates vasodilatation, platelets and neutrophils aggregation, and local organ blood flow. Therefore, NO influences cell and tissue function. The present study revealed that D-Gal induced hepatic injury was associated with elevated NO level. On the other hand, it has been shown that administration of NO donors provides cytoprotection from inflammatory liver damage and hepatocellular apoptosis.41 However, pharmacological blockade of iNOS either aggravates or attenuates liver injury or is ineffective.42 Interestingly, the present results showed that PTX as well as SYM reduced the total level of nitrate/nitrite (the stable metabolite of NO). Such reduction was significant compared to results of non pretreated D-Gal group.
Histological changes, especially acute inflammatory response was visible throughout many lobules. The present data suggest that PTX produced protective effects against the disturbed biochemical markers and histopathological findings of D-Gal induced hepatotoxicity in rat model. PTX reduced the levels of toxic free radical and attenuated NO production Thus, PTX increases hepatic antioxidant enzyme activities, and its antioxidant effect appears to play a key role in the attenuation of inflammation. The present results may indicate that PTX and SYM have an antioxidant effect on the increase of the SOD and CAT activities. The combination of PTX with SYM might not provide additional benefits when compared to the use of PTX or SYM alone in the maintenance of antioxidant defense of liver tissues.
In conclusion, these results indicate that PTX attenuated the hepatic damage-induced by D-Gal by reducing oxidative stress and increasing the antioxidant defense activities.
AcknowledgmentWe thanks Prof. Mohamed Hamid, Vet Medicine, Zagaig University, for his kind help in histopathology study.