Introduction and aim. Non-cirrhotic idiopathic portal hypertension (NCIPH), also known as hepatoportal sclerosis (HPS) is a disease of uncertain etiology. However, many pathophysiological mechanisms has been postulated, including thrombophilia, chronic recurrent infections and exposure to drugs or toxins. In this context, it appears to be of multifactorial etiology or resulting from a portal vascular endothelium aggression. It is important to consider whether the use of dietary supplements and herbs can trigger or contribute to the occurance of HPS. We report a possible association of HPS with the consumption of herbal and / or dietary supplements.
Material and methods. We describe two cases of HPS in patients without known etiology causes associated with this disease.
Results. Both patients were females who were diagnosed with HPS following the consumption of Herbalife® products and putative anorexigenic agents in the herbal infusions. Image-based analysis and the assessment of the histopathological alterations found in the livers confirmed the diagnosis. The histopatological analysis of liver samples from both patients showed portal tracts enlarged by fibrosis with disappearance or reduction in the diameter of the portal vein branches. In many portal tracts, portal veins branches were replaced by aberrant thin-walled fendiforme vessels. The bile ducts and branches of the hepatic artery show normal aspects.
Conclusion. After the exclusion of other etiologic factors and a comprehensive analysis of clinical history, consumption of Herbalife® products and anorexigenic agents was pointed-out as a puttative predisposing factor for the development of the disease.
Portal hypertension is a syndrome characterized by an increase of 5 mmHg or higher in the pressure gradient between the portal vein and the inferior vena cava.1,2 In the western countries, liver cirrhosis is the most frequent cause of portal hypertension.1
However, portal hypertension may also affect patients without parenchymal liver disease. In such cases, it is referred as non-cirrhotic portal hypertension.2 There are many causes of non-cirrhotic portal hypertension,2 including portal vein thrombosis,4 hepatic vein thrombosis, congenital defect of the portal plate, hepatosplenic form of schistosomiasis, sinusoidal obstruction syndrome, arteriovenous fistulas, obstruction of intrahepatic portal vessels.2
In many developing countries, the most common cause of non-cirrhotic portal hypertension is schistosomiasis.3,5 In Brazil, it is estimated that about six million people are infected with Schistosoma mansoni, whereas over 25 million are at risk. Of those, approximately 10% are in risk of developing portal hypertension.6 On the other hand, in developed countries vascular diseases of the liver assume greater importance.7
Among the diseases that make up the spectrum of the non-cirrhotic portal hypertension, obliterative portal venopathy (OPV) or hepatoportal sclerosis (HPS) stands out. As an entity of confused nomenclature3 it is also called non-cirrhotic portal fibrosis in India.8 In Japan and other Asian countries it is referred to as idiopathic portal hypertension9 while HPS is most commonly used in the US,10 Europe11 and Brazil.
HPS is an underdiagnosed and neglected disease. Its image exams and histopathological aspects are very similar to those of hepatosplenic schistosomiasis. Because of that, leads to diagnostic confusion between both diseases, notably in countries where schistosomiasis is endemic. Only recently, with the control of schistosomiasis and the expansion of liver transplant programs in Brazil, this disease acquired some importance in the context of non-cirrhotic idiopathic portal hypertension, but little is known about its etiology.
Despite various theories proposed for the development of HPS, there is still no defined etiology, but it is known that HPS may be seen in the setting of hypercoagulable states, collagen vascular diseases, and drug-induced liver injury.11,12 The histological hallmarks are: portal-septal fibrosis, narrowing of the portal lumen and/or obliteration of the small branches of the portal vein11 and the appearance of new aberrant portal lymphatic vessels.13,14
Nowadays there is a steady increase in the consumption of medicinal herbs and dietary supplements based on so called natural ingredients.15,16 Such products are frequently considered miraculous and harmless.17 Also, food supplement, such as Herbalife® products are highly consumed, although no robust studies about its safety and efficacy are available, so far.
Liver injury induced by several herbs and herbal cocktails has been widely communicated, with effects varying from asymptomatic elevations of hepatic enzymes to fulminant hepatites.18,19 Most of the times, adverse reactions are long term and that makes it difficult to establish a causality relationship between the use of such agents and the development of liver disease.20,21
Based on two case studies, this paper aims to suggest a possible association of hepatoportal sclerosis with the consumption of herbs and/or food supplements.
Material and MethodsTwo patients were approached with the diagnostic of HPS who had the history of consuming Herbalife® food supplement and herbal homemade infusions with a supposed anorexigenic activity.
Both patients were seen in a refferal center for the liver disease in Salvador-Bahia, northeast of Brazil, in june of 2014. The histologic evaluation was fulfilled at a single refferal center for liver pathology at Fiocruz: Gonçalo Muniz research center, in Salvador-Bahia, Brazil.
The following data was used in the investigation:
- •
Study of Medical Charts; Assessment of histopathological, image and biochemical exams.
- •
The Roussel Uclaf Causality Assessment Method (RUCAM) algorithm for causality assessment was used.
The current paper is included in the scope of Brazil’s hepatotoxicity assessment project headquartered at our institution, approved by the local Human Research Ethics Committee and registered at the Ministry of Health’s Plataforma Brasil system. Because it is a non-interventional study based on the review of liver biopsy slides and of information provided in medical charts, researchers declared full confidentiality about the data collected and they committed themselves to using such information for scientific purposes only, thus fully preserving the identities of patients.
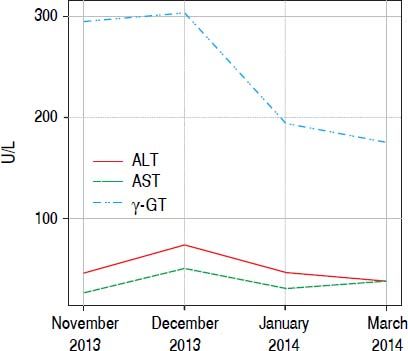
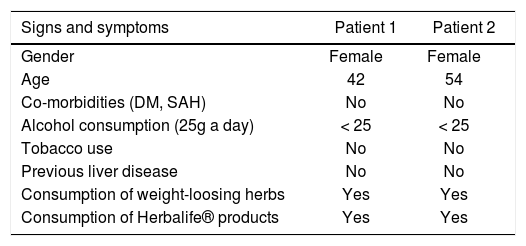
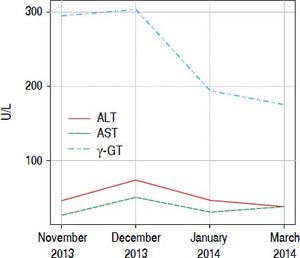
ResultsPatient No. 1A 42 years old female patient who came to our unit with complaints of asthenia and dizziness over the past week. In addition, the patient referred to postprandial fullness and abdominal discomfort. Patient reported use of Herbalife® products consumption for 3 months before coming to the hospital. During this time, it was observed increased serum levels of γ-glutamyltranspeptidase (γGT), reaching six times above the upper limite of the normality. After discontinuing the consumption of Herbalife® products there was an immediate improvement in the levels of liver enzymes, but aminotransferases and γGT persisted slightly elevated (Figure 1).
The patient denied the use of any other products or medications concomitantly to the above mentioned supplement; she referred a prior consumption of Herbalife® products six years ago, but she didn’t have any lab test done; she also mentioned the use of a phytotherapic commercialized with the name of a 30-ervas® tea and a tea called in Brazil “chá de cavalinha” (Equisetum giganteum L.) (Table 1), but she stopped to consume these infusions six months before starting the Herbalife® products. Besides, she reported to consume alcohol under 25g a day she denied to use tobacco and illicit drugs.
General characteristics of patients.
| Signs and symptoms | Patient 1 | Patient 2 |
|---|---|---|
| Gender | Female | Female |
| Age | 42 | 54 |
| Co-morbidities (DM, SAH) | No | No |
| Alcohol consumption (25g a day) | < 25 | < 25 |
| Tobacco use | No | No |
| Previous liver disease | No | No |
| Consumption of weight-loosing herbs | Yes | Yes |
| Consumption of Herbalife® products | Yes | Yes |
DM: diabetes mellitus. SAH: systemic arterial hypertension.
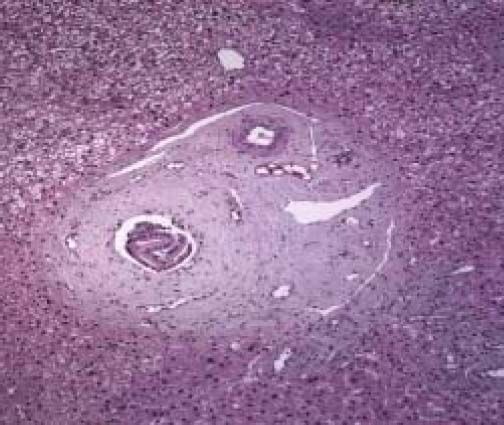
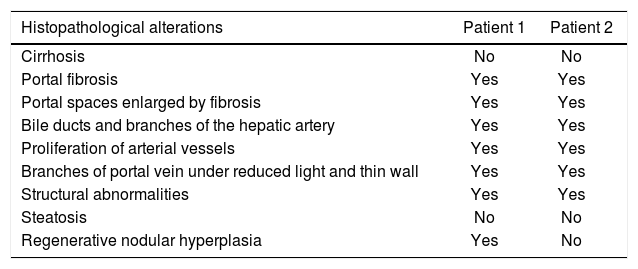
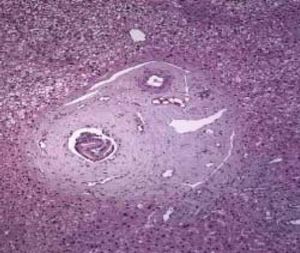
The physical exam was normal. The patient was submitted to liver biopsy and histology showed obliterative portal venopathy (hepatoportal sclerosis) with grade I regenerative nodular hyperplasia without evidence of biliary disease or hepatic steatosis (Figures 2 and 3; Table 2).
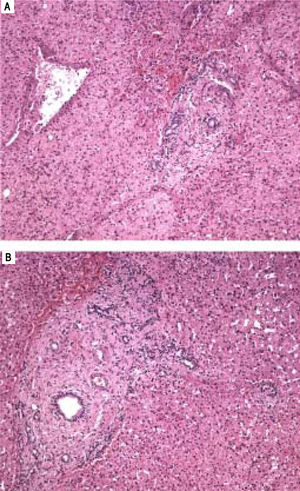
Histological features of hepatoportal sclerosis diagnosed on biopsies, patient 1. Portal spaces increased by fibrosis. The bile ducts and branches of the hepatic artery show norma aspects. The branches of the portal vein appear like fungiform vessels with reduced light and a very thin wall, without a muscular layer. The surrounding hepatic parenchyma is norma (Hematoxylin and eosin x 100).
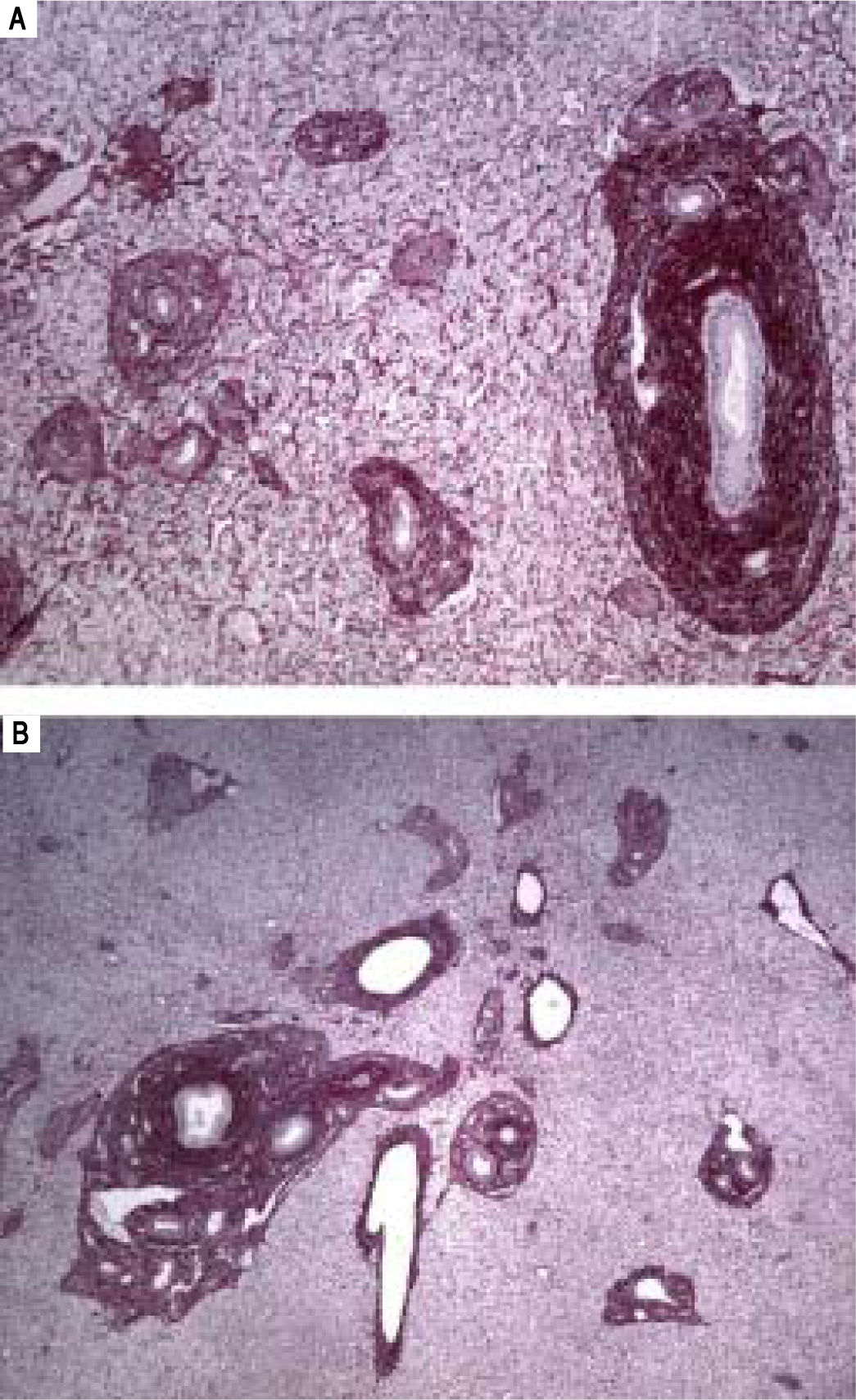
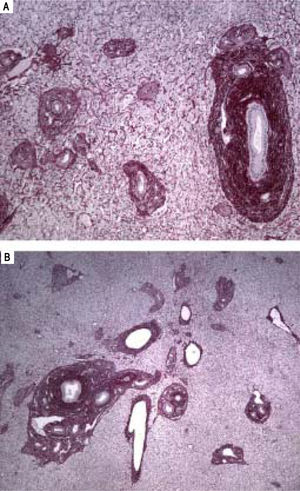
A-B. Histological features of hepatoportal sclerosis diagnosed on biopsies, patient 1. Cross-section of liver tissue showing approximation of vascular structures (portal space sand central veins) thus reflecting the extinction of the hepatic parenchyma. Additionally, in figure 3B, discreet per sinusoidal fibrosis is observed. The branches of the portal vein are either absent in portal spaces or reduced to slotted structures of thin walls (red picrosirus counterstained with hematoxylin, x40 in 2A and x20 in 2B).
Histological features observed on biopsies.
| Histopathological alterations | Patient 1 | Patient 2 |
|---|---|---|
| Cirrhosis | No | No |
| Portal fibrosis | Yes | Yes |
| Portal spaces enlarged by fibrosis | Yes | Yes |
| Bile ducts and branches of the hepatic artery | Yes | Yes |
| Proliferation of arterial vessels | Yes | Yes |
| Branches of portal vein under reduced light and thin wall | Yes | Yes |
| Structural abnormalities | Yes | Yes |
| Steatosis | No | No |
| Regenerative nodular hyperplasia | Yes | No |
Additional investigation revealed negative serology for the following viruses: hepatitis A, B, C, cytomegalovirus (CMV), Epstein Barr virus (EBV), human immunodeficiency virus (HIV) and human T-cell lymphotropic virus (HTLV). The antibodies (antinuclear antibody, anti-smooth muscle antibodies and ant mitochondrial) were negative.
The thrombophilia tests were also negative for the mutation of Jak 2/Tetrahydrofolate dismutase/prothrombin/ Layden V factor. Tests for homocysteine, antithrombin III, protein C and protein S deficiency were all normal. Electrophoresis of protein was normal, skin test for schistosoma was negative and parasitological stool test was negative for Schistosoma mansoni eggs. Abdominal ultrasound was normal.
No description of interactions among the different components was found in the literature to suggest interaction between Herbalife® products and infusions taken by the patient. According to the RUCAM algorithm, causality is highly likely for this patient because of the temporal relationship, the exclusion of other causes and, although not part of the RUCAM criteria, histology shows liver injury.
Patient No. 2A 54 years, female patient, Caucasian, reported the use of the Herbalife® products throughtout 2013, During this period, she also used the 30-ervas® tea and another one that has a slimming effect, sometimes concomitantly. All of them were self-precribed based on information found on the internet. In 2013, the patient had a bariatric surgery. During the procedure neither a liver biopsy was performed, nor a macroscopic aspects of the liver was detailed.
Six months after surgery, the patient started to a discomfort on the right hypochondrium, nausea and vomiting. In the emergency room, the patient was taken for a Cholecystectomy due to acute cholecyste. During the laparoscopy a liver biopsy was performed. The histopathology showed hepatoportal sclerosis. The patient denied previous liver diseases or other comorbidities. She reported alcohol consumption of < 25 g/of alcohol a day and she denied tobacco or illegal drugs use (Table 1).
Regarding the physical examination, the patient showed good nutrition status and overall wellness, with blood pressure (PB) of 120 × 60 mmHg. The liver was palpable at 6 cm from the right costal edge and 10 cm from xiphoid appendix, but without splenomegaly. No stigmata of chronic liver disease was found.
The laboratory tests for screening autoimmune, infectious and parasitic liver diseases were negative. The genetic thrombophilia, proteins C and S, antithrombin III, prothrombin gene mutation, Leiden V-factor mutation, Jak 2 mutation, Ham test, and homocysteine were normal. The liver biochemistry profile was normal too. Further investigation revealed negative serology for human immunodeficiency virus (HIV).
In the absence of a diagnosis, complementary exams were requested: abdominal ultrasound revealed increased liver echogenicity suggesting periportal fibrosis; magnetic resonance imaging (MRI) revealed compatibility with chronic parenchymatous liver disease with no dilation of biliary ducts. The endoscopy only revealed reflux esophagitis. The stool parasitological exam was negative for Schistosoma mansoni eggs and the skin test for schistosomiasis was also negative.
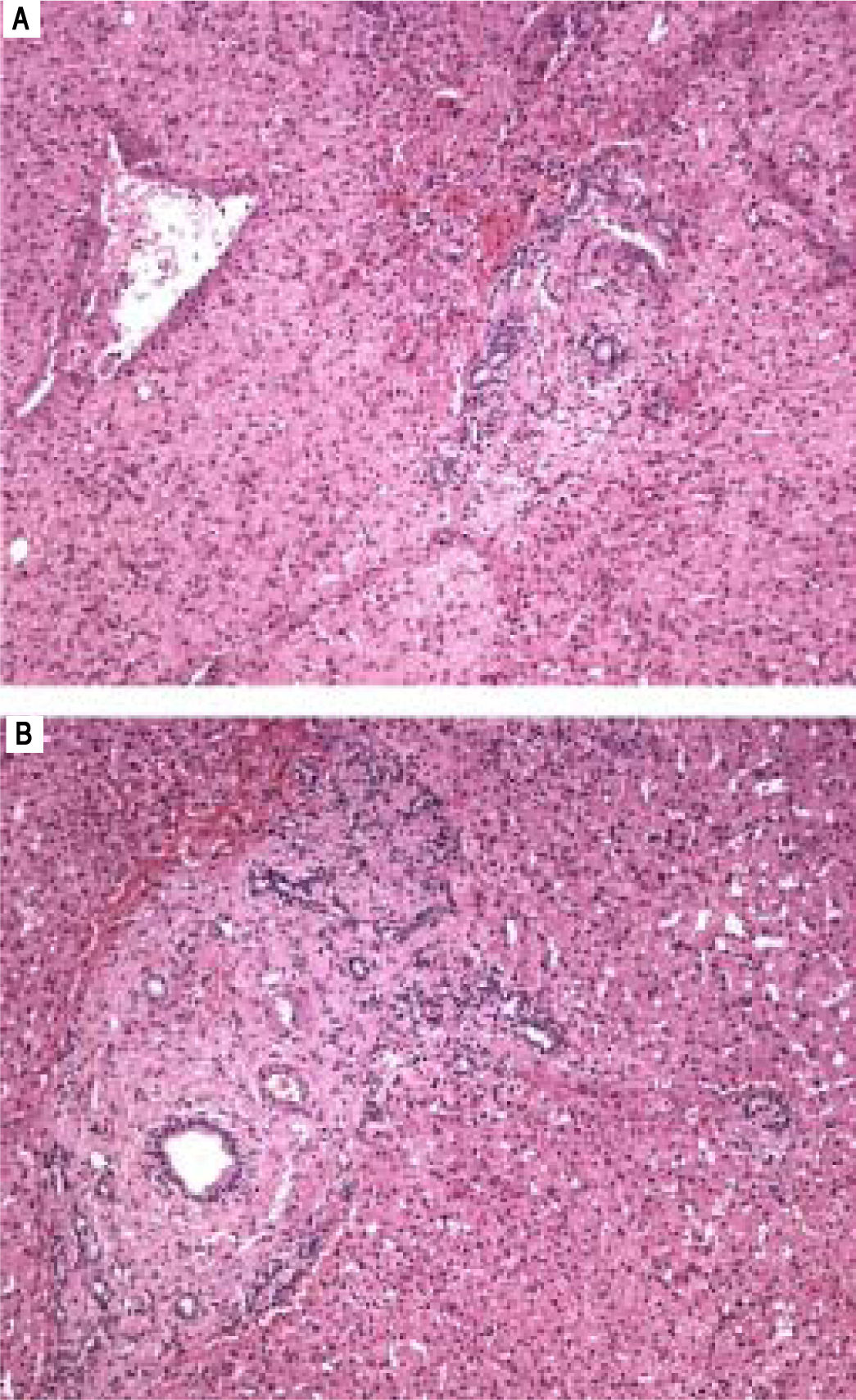
Liver biopsy showed alterations that are characteristic of HPS (Figure 4 and Table 2). The causality relationship was classified as possible according to the RUCAM algorithm. Therefore, with the exclusion, after ruling out other etiologic factors and cautiously assessing the clinical history, the consumption of Herbalife® products and weight-losing teas was pointed out as a possible predisposing factor for the development of the disease.
A.Histological features of hepatoportal sclerosis diagnosed on biopsies, patient 2. Portal space enlarged by fibrosis with the disappearance of the branches of the portal vein. Other elements of the portal triad (bile ducts and branches of the hepatic artery) are preserved (Hematoxylin and eosin x100). B. Portal space enlarged by fibrosis with the disappearance of the branches of the portal vein. There is approximation of the central vein, thus suggesting extinction of hepatic parenchyma (hematoxylin and eosin x40).
The current study reported two cases of patients with HPS confirmed by liver biopsy. The patients profile do not indicate any genetic thrombophilia. No autoimmune disease, infectious diseases, hematologic or exposure to immunosuppressants or chemotherapy drugs were reported. However, a careful interview pointed out the common use of Herbalife® products and others based on plants herbs.
Schistosomiasis was also discarded due the absence of epidemiological data which suggested the exposure to this parasite. Besides that, the skin test for schistosoma and parasitological stool were both negative for Schistosoma mansoni eggs.
Our hypothesis is that hepatoportal sclerosis could result from hepatotoxicity, since our study reports two female patients with HPS who were exposed to the Herbalife® products and other products based on plants. Both diseases, drug induced liver disease (DILI) and HPS are more common in female patients over 40 years old, as reported in both presented cases.22,23
The DILI hypothesis seems very plausible in patient 1. In this patient, the toxicity by Herbalife® products finds a strong temporal connection, because during the period of the use of the product; it was possible to document the increase of the γGT enzyme six times upper the normal value, and these values decayed with the interruption of the product. The same happened with the aminotransferase. The hystopatological study showed showed findings compatible to HPS.
Other products such as 30-ervas® and Equisetum giganteum teas could also be blamed as responsible for either the onset or the progression of the liver injury, but, because their consumption was stopped six months before the beginning of Herbalife® product utilization, their supposed etiology became less likely. There was no concomitant use of any Allopathic drug that could be implicated in the pathogenesis of this disease. As usually happens, during the previous exposure to these products, no test for liver injury had been performed. Then, we can assume that the vast majority of liver injury in cases like this one are not diagnosed, which means a high probability of unreported cases.
The patient 2, differently from the patient 1, had the consumption of Herbalife® products concomitantly with the use of 30-herb and weight-losing teas. Therefore, we cannot state that those teas either caused or contributed to the development of the liver injury. In this particular case, the liver biochemistry profile was normal. However, computed tomography (CT) imaging detected and liver biopsy confirmed liver injury compatible with HPS.
Clinical interpretation is a first important step taken toward the understanding of any liver disease as being caused by a drug or toxin. The RUCAM scale is most widely accepted for hepatotoxicity causality assessment for its ability to assign a final diagnosis of drug-induced hepatotoxicity without rechallenge patients.24
For the above cases, RUCAM scores were 8 (highly likely diagnosis) for patient 1 and 4 (possible) for patient 2. The result of the assessment according to the RUCAM algorithm may be explained by the low specificity for the evaluation of adverse events between the consumption of herbal medicines or food supplements and the mechanism of certain reactions. Therefore, in such cases, clinical assessment is superior for differential diagnosis.
DILI associated with the consumption of Herbalife® products was reported for the first time in Switzerland and in Israel.15,26 Then, other studies in Spain, Argentina, and Iceland reported the potential hepatotoxicity of these products.25,27–31 There are already seven published papers that reported 54 cases of liver injury related to the use of Herbalife® products.15
However, there is no proved association with the specific development of HPS as a DILI, there are extremely important elements that support the possibility of these products cause many types of liver injury, including vascular disease.
It is known that some herbs are definitely associated with other vascular liver diseases which enhances the hypothesis in question in our study. In this context, Pyrrolizidine alkaloidosis that is included in some herbal preparations such as comfrey (Symphytum officinale), causes injury on the vascular endothelium of the centerlobular vein, and, as a consequence, the hepatic veno-occlusive disease develop.32–34
Substantial contribution to this potentially hazardous situation includes nutritional supplements such as vitamins, antioxidants, herbs and complementary formula to induce weight loss and “shape up the body”.20 Most of the times, the consumption of herbs and food supplements takes place as self-medication, without a prior visit or the consent of a doctor.20 In addition, incidence is underestimated due to patients underreporting26 as well as the absence of specific symptoms.
Histopathological findings that stood out in our reported cases were: portal spaces enlarged by fibrosis, whereas bile ducts and branches of the hepatic artery displayed normal aspects; branches of the portal vein showing slotted vessels, light reduced and with a thin wall; approximation of vascular structures (portal spaces, central veins). Furthermore, the approximation of the central vein could reflect extinction of the liver parenchyma.
Similar findings have been described in several other studies.35–38 These aberrant vessels may be dilated lymphatic structures, due to.39 However, another hypothesis suggests that the trigger acts directly on the sinusoidal wall and the wall of the vein to induce fibrosis, obstruction, and minor architecture changes.40–42
There is no clear and definitive explanation of the pathophysiology of EHP, but it is believed that the vascular changes are triggered by a prothrombotic disorder, which causes obstruction of large and small vessels.43 Faced with various speculations about the etiology of HPS, it is likely to be a multifactorial disease, however, thrombotic disorders are fundamental to the development of the disease.
In conclusion, despite of the complexity in defining the etiological factor responsible for HPS, the hypothesis this liver disease induced by food supplements and/or herbal medicines seems to be probable in these cases.
The presence of liver abnormalities, either in laboratory tests, imaging or histology in patients that have reported use of these products should be considered as a differential diagnosis HPS.
Prospective cohort studies are necessary to better characterize alterations in liver enzymes as well as histological alterations in patients who have been exposed to herbs and food supplements, with the objective of establishing a causal nexus.
AcknowledgmentThis paper was supported by Hepatox Study Group financed by Johnson & Johnson Fundation, as well as by Maria Emília Fundation, Bahia, Brazil.
Abbreviations- •
γGT: glutamyltranspeptidase.
- •
ALT: alanine-aminotransferase.
- •
AST: aspartate-aminotrans-ferase.
- •
CMV: cytomegalovirus.
- •
CT: computed tomography.
- •
DILI: drug-induced liver injury.
- •
DM: diabetes mellitus.
- •
EBV: Epstein Barr virus.
- •
HIV: human immunodeficiency virus.
- •
HPS: hepatoportal sclerosis.
- •
HTLV: human T-cell lymphotropic virus.
- •
NCIPH: non-cirrhotic idiopathic portal hypertension.
- •
OPV: obliterative portal venopathy.
- •
PB: blood pressure.
- •
RUCAM: Roussel Uclaf Causality Assessment Method.
- •
SAH: systemic arterial hypertension.