Introduction. Hepatocellular carcinoma (HCC) has become a frequent type of cancer in Mexico. At the present time it represents the 19th cause of death in the population.
Objective. To recognize the epidemiological profile and the treatment results in a cohort of federal employees with HCC.
Material and methods. We analyzed 47 consecutive cases with HCC diagnosis from January 2004 till December 2007. Twenty four demographic data, tumor staging, clinical, and biochemical variables were analyzed to identify parameters predicting survival by computing Kaplan-Mier and Mantel-Cox survival curves.
Results. Patient reference increased 5% each year. The mean age was 60.4 years, 63.8% female sex, and 72.3% had cirrhosis, 44.7% had Hepatitis C infection. Most patients presented with advanced disease: 55.3% were AJCC stage 3 and 21.3% stage 4, 51.1% were BCLC class D. Mean tumor size was 8.09 cm. Median survival time from diagnosis was 122 days. Patients that did not receive treatment had a median survival of 70 days; the longest survival of patients was of those that received transarterial chemoembolization with a median of 707 days, followed by surgery with 683 days. Univariate analysis showed survival was associated to MELD score, AJCC and BCLC staging, creatinine level and ascites. Multivariate analysis showed tumor differentiation, AJCC staging and the choice of treatment to be related to the risk of death.
Conclusion. An increase in the referral of HCC was demonstrated. Most patients had cirrhosis and HCV infection. Due to advance disease staging, TACE was the treatment that offered longest survival.
The increasing incidence and mortality of Hepatocellular Carcinoma (HCC) is recognized worldwide.1-3 In the Latin-American and other developing countries, this malignant tumor has a poor prognosis since tumors are diagnosed when surgery is not feasible, either the tumor is significantly large or cirrhosis is too advanced.4 As a rule, HCC life expectancy is measured in weeks or months. At the present time HCC is among the 20 top causes of general death in Mexico.5,6 Traditionally, the Mexican health system reports data on mortality trends. Incidence is not generally informed, that represents a predicament to calculate the real effect of HCC or any other disease. Regardless of this fact, few studies have explored HCC epidemiological profile; three of these studies have been set in Mexico City at medical centers and other study in a university hospital on the north of Mexico. These studies have been retrospective and patient’s data have been collected in time frames that vary from 10 to 25 years.7-10
In the last decade HCC management has changed worldwide and many treatment modalities have been proved and validated.11-13 New drugs have been developed and demonstrated to be successful in controlling this aggressive disease.14 However, in our country another void is the knowledge of actual treatment options offered to patients with HCC. This is consequence of two key interactions, first is that few patients have access to specialized care centers where HCC treatment options are available. Second is that technology is expensive and not widely available in our healthcare system.
The objective of our study was to compile the epidemiological profile of the patients with HCC and analyze the treatment and variables related to outcome in a cohort of 47 patients that were send to the “Centro Medico 20 de Noviembre” in Mexico City from 2004 to 2007.
Material and MethodsFrom 2004 through 2007 forty seven patients diagnosed with primary liver tumors were sent to our hospital to be staged and offered a treatment, including liver transplantation. Patients with diagnosis of solitary masses smaller than 5 cm without portal hypertension were first evaluated for liver resection. Patients within Milan criteria were evaluated to be transplant candidates. Three patients on the liver transplant waiting list developed tumors and were included in this analysis. All other patients were evaluated to receive a palliative treatment either radiofrequency ablation (RFA), Transarterial chemoembolization with doxorubicin (TACE), chemotherapy or the best palliative care available. Centro Medico Nacional “20 de Noviembre” is a government-based urban medical center that receives patients from all Mexico. Coverage includes federal employees and its economic dependents.
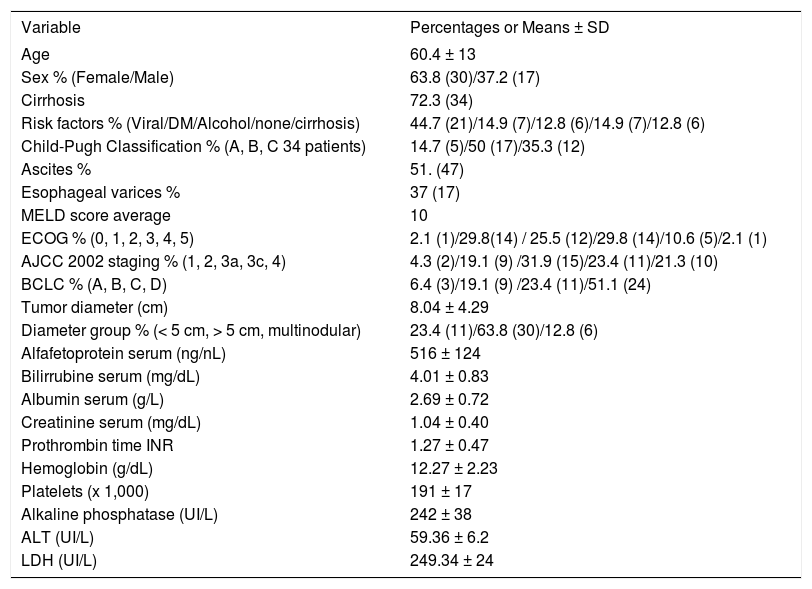
Characteristics of the patientsThe baseline characteristics are presented in table 1 The mean patient age was 60.4 years old (range 35-86 years) and predominantly of female sex (63.8%) and cirrhotic (72.3%) half of the patients were Child-Pugh B with an average MELD scoring of 10. Forty four percent of patients had positive serology for Hepatitis C infection, 14.9% diabetes mellitus and 14.8% had no risk factor associated to HCC. Tumor staging was calculated in the American Joint Committee on Cancer 2002 tumor-node-metastasis system (AJCC) and Barcelona Clinic Liver Cancer Staging System (BCLC); most patients were in advanced disease as is shown in table 1. Only 23.4% tumors were smaller than 5 cm, while 63.8 % were > 5 cm, and 12.8% of tumors were multifocal, mean tumor size was 8.09 cm (SD ± 4.2) Ascites was present in 51.1% at time of diagnosis, but only 37% had esophageal varices documented. Mean alpha-fetoprotein (AFP) serum level was 516 ng/mL, mean values of bilirrubin was 4.07 mg/dL, and albumin 2.6 g/L, other biochemical and blood count values are shown in table 1. Biopsy was feasible in 57% of patients and in the largest part the histology report was of trabecular carcinoma (62.2%).
Characteristics from 47 patients with hepatocellular carcinoma Data presented as percentages (patient number inside parentheses) and average ± SD.
| Variable | Percentages or Means ± SD |
|---|---|
| Age | 60.4 ± 13 |
| Sex % (Female/Male) | 63.8 (30)/37.2 (17) |
| Cirrhosis | 72.3 (34) |
| Risk factors % (Viral/DM/Alcohol/none/cirrhosis) | 44.7 (21)/14.9 (7)/12.8 (6)/14.9 (7)/12.8 (6) |
| Child-Pugh Classification % (A, B, C 34 patients) | 14.7 (5)/50 (17)/35.3 (12) |
| Ascites % | 51. (47) |
| Esophageal varices % | 37 (17) |
| MELD score average | 10 |
| ECOG % (0, 1, 2, 3, 4, 5) | 2.1 (1)/29.8(14) / 25.5 (12)/29.8 (14)/10.6 (5)/2.1 (1) |
| AJCC 2002 staging % (1, 2, 3a, 3c, 4) | 4.3 (2)/19.1 (9) /31.9 (15)/23.4 (11)/21.3 (10) |
| BCLC % (A, B, C, D) | 6.4 (3)/19.1 (9) /23.4 (11)/51.1 (24) |
| Tumor diameter (cm) | 8.04 ± 4.29 |
| Diameter group % (< 5 cm, > 5 cm, multinodular) | 23.4 (11)/63.8 (30)/12.8 (6) |
| Alfafetoprotein serum (ng/nL) | 516 ± 124 |
| Bilirrubine serum (mg/dL) | 4.01 ± 0.83 |
| Albumin serum (g/L) | 2.69 ± 0.72 |
| Creatinine serum (mg/dL) | 1.04 ± 0.40 |
| Prothrombin time INR | 1.27 ± 0.47 |
| Hemoglobin (g/dL) | 12.27 ± 2.23 |
| Platelets (x 1,000) | 191 ± 17 |
| Alkaline phosphatase (UI/L) | 242 ± 38 |
| ALT (UI/L) | 59.36 ± 6.2 |
| LDH (UI/L) | 249.34 ± 24 |
DM: Diabetes mellitus. ECOG: Eastern Cooperative Oncology Group. AJCC02: American Joint Commission on Cancer 2002. BCLC: Barcelona Clinic Liver Cancer. ALT: Alanine aminotransferase. DHL: Lactic Dehydrogenase (Staging Systems: please refer to table 3).
All patients were followed-up monthly or bi-monthly with complete blood count, biochemistry and liver functions test until death. If alive, AFP and hepatic Computed Tomography scan (CT) were performed every six months to assess tumor progression and treatment results. Patients with liver decompensation were hospitalized to receive care. Deaths from patients living outside Mexico City were confirmed by telephone call of the investigators, but some details were missing and it was decided not to include cause of death since sometimes it was difficult to confirm.
Statistical analysisThe baseline characteristics of patients are reported in means ± SD or percentages. Follow-up is expressed in average days. Mean and median survivals are expressed in days.
The univariate analysis to identify parameters predicting survival was performed by computing survival curves according to the Kaplan-Meier method and comparing them by the Mantel-Cox test.
Twenty-four baseline variables were assessed in the study: age, sex, ascites, cirrhosis, diabetes mellitus, alcoholism, viral infection, Child-Pugh classification, MELD score, esophageal varices, Eastern Cooperative Oncology Group Performance Status (ECOG) American Joint Committee on Cancer 2002 tumor-node-metastasis system (AJCC) and Barcelona Clinic Liver Cancer Staging System (BCLC), bilirubin, serum albumin, prothrombin INR, serum creatinine, hemoglobin, platelets, ALT, alkaline phosphatase, AFP, lactic deshydrogenase (LDH), tumor type and differentiation and treatment prescribed.
Those variables significantly related to survival in the univariate analyses (p<0.05) or close to significance (p<0.1) were subsequently included in a step-wise Cox regression analysis for identification of independent predictors of mortality.
All the calculations were performed by using the SPSS statistical package (SPSS, Inc., 1989-1995, Chicago, IL).
ResultsFrom the 47 patients included in this study, 23.4% (11 patients) were sent in 2004, 19.1% in 2005, 27.7% in 2006 and 29.8% in 2007, respectively. A small but significant increasing trend was noted (p = 0.000). Not any patient diagnosed in 2004 was alive at the end of the follow-up (December 2007), while 9.1% (one patient), 18.2 % (two patients) and 72.7% (eight patients) diagnosed in 2005, 2006 and 2007 were alive at the conclusion of this study (p = 0.002)
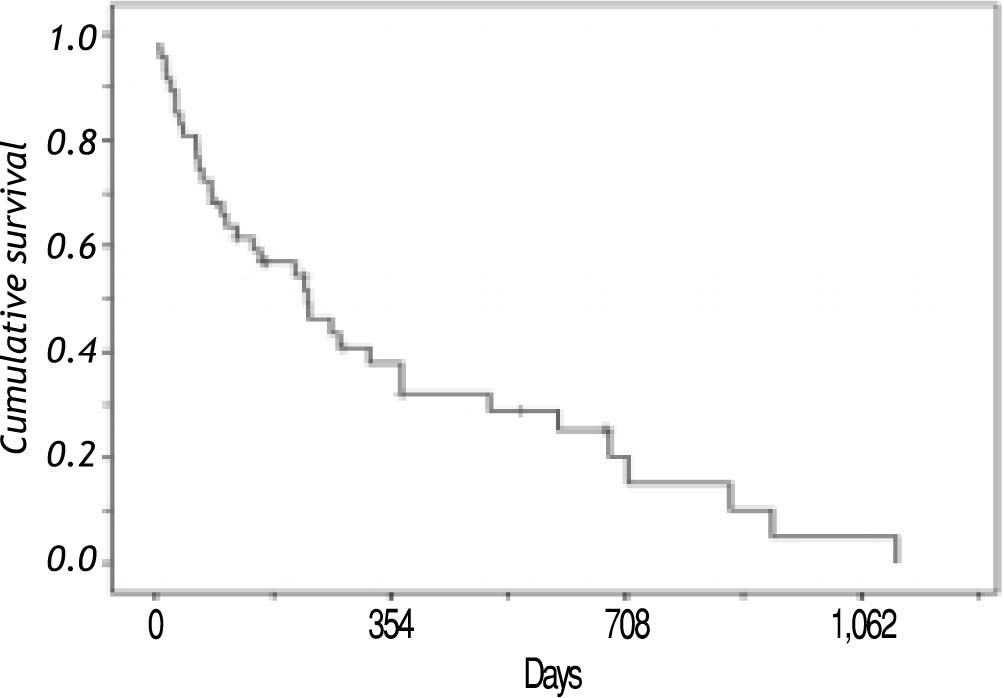
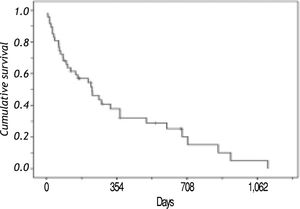
SurvivalAt the end of the follow-up, the mean and median survival times from diagnosis were 269 and 163 days in that order, with a range from one day to 1,114 days. The overall probability of survival at one year was 40%, at 18 months were 28% and at 36 months were 5%. Thirty six patients died (76.5%) during follow-up (Figure 1).
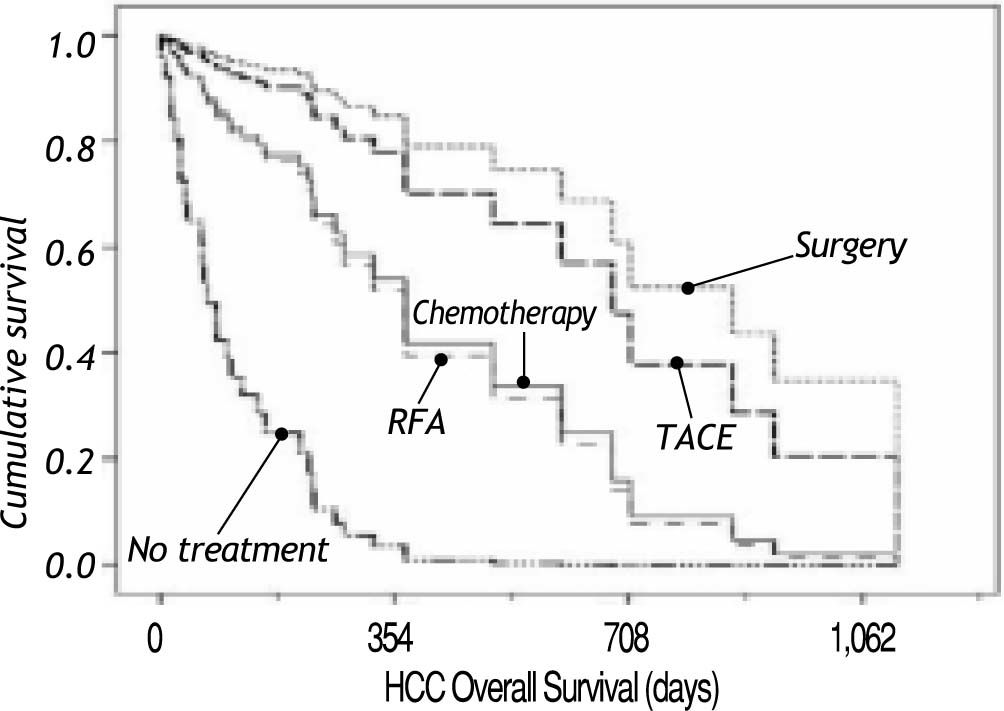
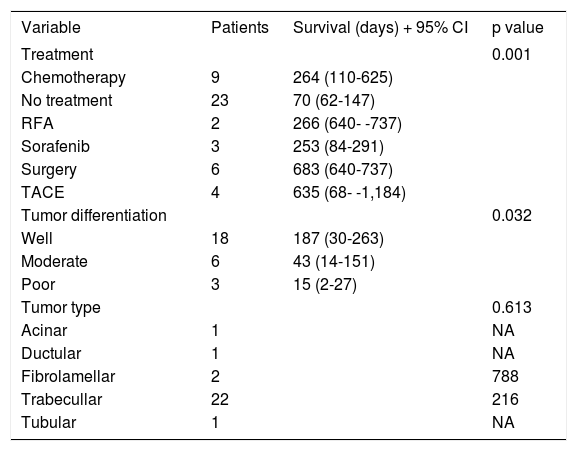
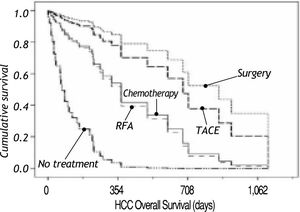
Figure 2 shows survival associated to treatment option. Patients that did not receive any treatment had a median survival of 70 days; the longest median survival of those that received treatment was those that received TACE with a median of 707 days, followed by surgery with a median survival of 683 days. Patients that received chemotherapy had a median survival of 264 days. Recently, new agents have received FDA approval as HCC treatment and Sorafenib (Nexavar®) has become available. A small group (six patients) has been prescribed with this drug. Median survival on Sorafenib (Nexavar®) currently is 253 days; up to now the median survival is similar to conventional systemic chemotherapy however it is too early to be compared with other chemotherapy treatment options available in our institution.
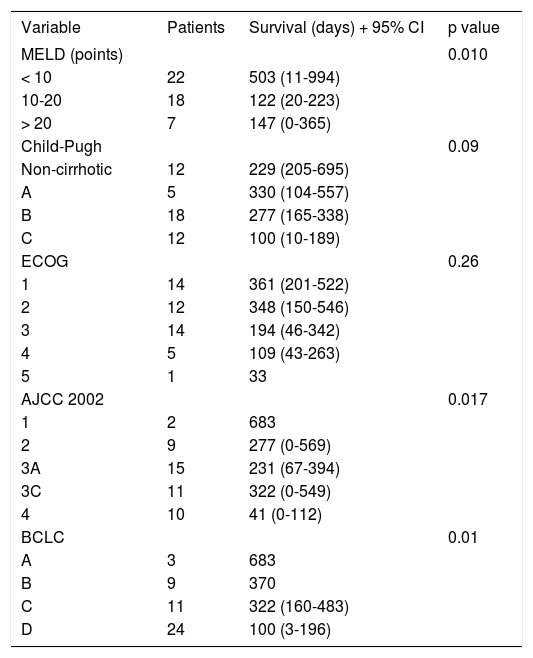
Prognostic FactorsFrom the 24 variables included, nine were associated to survival in the univariate analysis. Table 2 shows MELD, AJCC and BCLC system correlation to survival. Specifically, MELD Score System has been used to predict mortality in late stage liver disease and it was expected to be correlated to survival. A lower MELD score was correlated with a longer survival (p = 0.010). Child-Pugh class was closely associated to survival in the univariate analysis (p = 0.09). Tumor staging systems AJCC2002 and BCLC were found to be correlated to survival as lower scoring was associated to a longer survival. Early stages as BCLC A or AJCC2002 stage 1 had the longer survival (median of 683 days) compared with later stages as BCLC D (median of 100 days, p = 0.010) or AJCC2002 Stage 4 (median of 41 days, p = 0.017)
Survival by staging system. ECOG: 0 fully active, 1 Restricted in physically strenuous activity but ambulatory and able to carry out work, 2: Ambulatory and capable of all self-care but unable to carry out any work activities, 3: Ambulatory and capable of all self-care but unable to carry out any work activities, 4: Completely disabled, 5: Dead (as published in Am J Clin Oncol 1982; 5: 649-55). AJCC02: American Joint Commission on Cancer 2002. Stage 1: Solitary tumor without vascular invasion. Stage 2: Solitary tumor with vascular invasion or multiple tumors none more than 5 cm. Stage 3a: Multiple tumors more than 5 cm or tumor involving a major branch of the portal or hepatic veins. Stage 3c: Any size of tumor with regional lymph nodes metastasis. Stage 4: Any size tumor with distal metastasis. BCLC: Barcelona Clinic Liver Cancer. 0: Very early, solitary lesion less than 2 cm and clinical stage Child-Pugh A, without Portal Hypertension. A: Early, solitary or less than 3 lesions size > 3 cm clinical stage Child-Pugh A-B with Portal Hypertension. B: Intermediate, multinodular tumor clinical stage Child-Pugh A or B and Portal Hypertension. C: Advanced, tumor with portal invasion and/or metastasis, Child-Pugh A or B. D: Terminal: advanced tumor on Child-Pugh C clinical stage.
| Variable | Patients | Survival (days) + 95% CI | p value |
|---|---|---|---|
| MELD (points) | 0.010 | ||
| < 10 | 22 | 503 (11-994) | |
| 10-20 | 18 | 122 (20-223) | |
| > 20 | 7 | 147 (0-365) | |
| Child-Pugh | 0.09 | ||
| Non-cirrhotic | 12 | 229 (205-695) | |
| A | 5 | 330 (104-557) | |
| B | 18 | 277 (165-338) | |
| C | 12 | 100 (10-189) | |
| ECOG | 0.26 | ||
| 1 | 14 | 361 (201-522) | |
| 2 | 12 | 348 (150-546) | |
| 3 | 14 | 194 (46-342) | |
| 4 | 5 | 109 (43-263) | |
| 5 | 1 | 33 | |
| AJCC 2002 | 0.017 | ||
| 1 | 2 | 683 | |
| 2 | 9 | 277 (0-569) | |
| 3A | 15 | 231 (67-394) | |
| 3C | 11 | 322 (0-549) | |
| 4 | 10 | 41 (0-112) | |
| BCLC | 0.01 | ||
| A | 3 | 683 | |
| B | 9 | 370 | |
| C | 11 | 322 (160-483) | |
| D | 24 | 100 (3-196) |
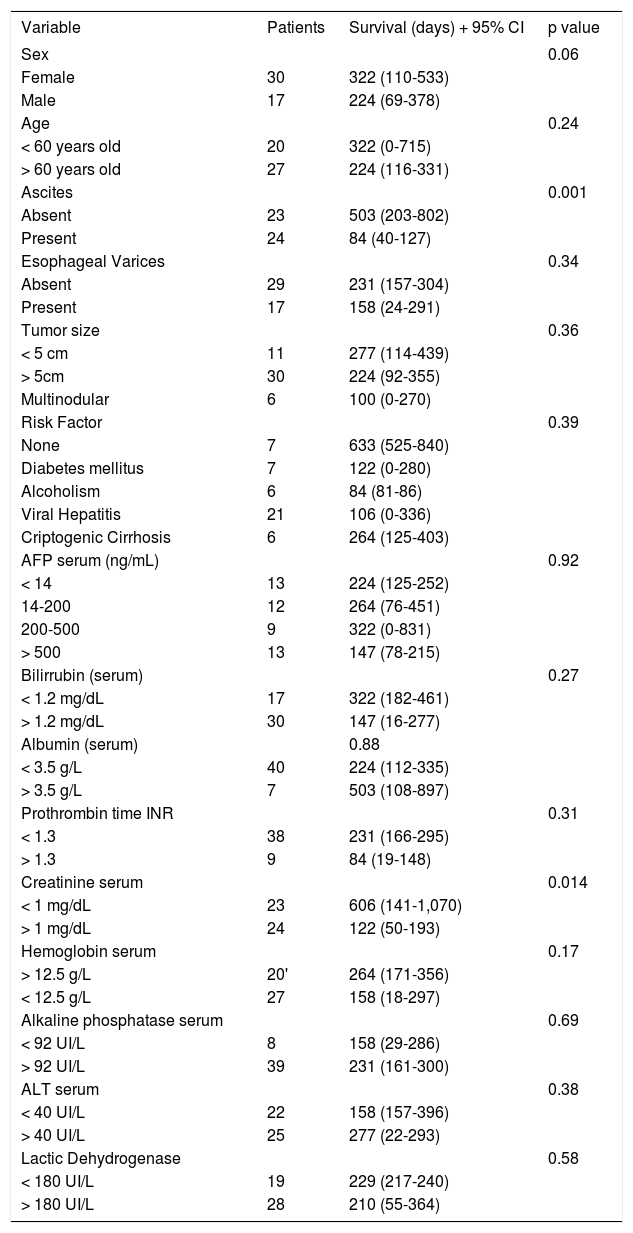
Table 3 shows clinical and biochemical variables. Only ascites (p = 0.001) and serum creatinine (p = 0.014) were significant associated with survival. Age and serum hemoglobin showed a trend to be significant (p = 0.06 and 0.17, respectively) and were included in the multivariate analysis. Both, treatment and tumor differentiation were associated with survival (Table 4). Patients treated with surgery and TACE had the longer survival (mean of 683 days and 635 days correspondingly, p value 0.001) compared with no treatment or conventional chemotherapy (264 and 70 days, respectively). Sorafenib (Nexavar®) has just recently been added to treatment options and the data on these results may not completely represent the outcome of treatment.
Univariate analysis on clinical and biochemical variables.
| Variable | Patients | Survival (days) + 95% CI | p value |
|---|---|---|---|
| Sex | 0.06 | ||
| Female | 30 | 322 (110-533) | |
| Male | 17 | 224 (69-378) | |
| Age | 0.24 | ||
| < 60 years old | 20 | 322 (0-715) | |
| > 60 years old | 27 | 224 (116-331) | |
| Ascites | 0.001 | ||
| Absent | 23 | 503 (203-802) | |
| Present | 24 | 84 (40-127) | |
| Esophageal Varices | 0.34 | ||
| Absent | 29 | 231 (157-304) | |
| Present | 17 | 158 (24-291) | |
| Tumor size | 0.36 | ||
| < 5 cm | 11 | 277 (114-439) | |
| > 5cm | 30 | 224 (92-355) | |
| Multinodular | 6 | 100 (0-270) | |
| Risk Factor | 0.39 | ||
| None | 7 | 633 (525-840) | |
| Diabetes mellitus | 7 | 122 (0-280) | |
| Alcoholism | 6 | 84 (81-86) | |
| Viral Hepatitis | 21 | 106 (0-336) | |
| Criptogenic Cirrhosis | 6 | 264 (125-403) | |
| AFP serum (ng/mL) | 0.92 | ||
| < 14 | 13 | 224 (125-252) | |
| 14-200 | 12 | 264 (76-451) | |
| 200-500 | 9 | 322 (0-831) | |
| > 500 | 13 | 147 (78-215) | |
| Bilirrubin (serum) | 0.27 | ||
| < 1.2 mg/dL | 17 | 322 (182-461) | |
| > 1.2 mg/dL | 30 | 147 (16-277) | |
| Albumin (serum) | 0.88 | ||
| < 3.5 g/L | 40 | 224 (112-335) | |
| > 3.5 g/L | 7 | 503 (108-897) | |
| Prothrombin time INR | 0.31 | ||
| < 1.3 | 38 | 231 (166-295) | |
| > 1.3 | 9 | 84 (19-148) | |
| Creatinine serum | 0.014 | ||
| < 1 mg/dL | 23 | 606 (141-1,070) | |
| > 1 mg/dL | 24 | 122 (50-193) | |
| Hemoglobin serum | 0.17 | ||
| > 12.5 g/L | 20' | 264 (171-356) | |
| < 12.5 g/L | 27 | 158 (18-297) | |
| Alkaline phosphatase serum | 0.69 | ||
| < 92 UI/L | 8 | 158 (29-286) | |
| > 92 UI/L | 39 | 231 (161-300) | |
| ALT serum | 0.38 | ||
| < 40 UI/L | 22 | 158 (157-396) | |
| > 40 UI/L | 25 | 277 (22-293) | |
| Lactic Dehydrogenase | 0.58 | ||
| < 180 UI/L | 19 | 229 (217-240) | |
| > 180 UI/L | 28 | 210 (55-364) |
Survival univariate analysis by treatment received and tumor differentiation.
| Variable | Patients | Survival (days) + 95% CI | p value |
|---|---|---|---|
| Treatment | 0.001 | ||
| Chemotherapy | 9 | 264 (110-625) | |
| No treatment | 23 | 70 (62-147) | |
| RFA | 2 | 266 (640- -737) | |
| Sorafenib | 3 | 253 (84-291) | |
| Surgery | 6 | 683 (640-737) | |
| TACE | 4 | 635 (68- -1,184) | |
| Tumor differentiation | 0.032 | ||
| Well | 18 | 187 (30-263) | |
| Moderate | 6 | 43 (14-151) | |
| Poor | 3 | 15 (2-27) | |
| Tumor type | 0.613 | ||
| Acinar | 1 | NA | |
| Ductular | 1 | NA | |
| Fibrolamellar | 2 | 788 | |
| Trabecullar | 22 | 216 | |
| Tubular | 1 | NA |
RFA: Radiofrequency ablation. TACE: Transarterial chemoembolization. NA: Not available.
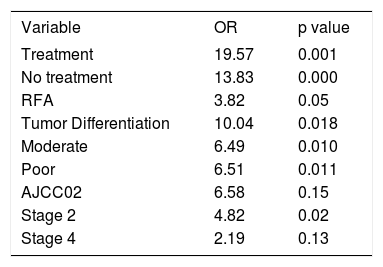
Multivariate analysis showed three independent variables predictive for mortality: tumor differentiation, AJCC02 stage and the choice of treatment. As it is seen in table 5, patients that did not received treatment or RFA had an increased risk of dying sooner than patients receiving any other treatment (OR 13.83, p = 0.000 and OR 3.82, p = 0.05, respectively). As expected, moderate or poorly differentiated tumors showed an increased risk of death since tumor differentiation is known to be an indicator associated to tumor’s aggressive behavior and poor prognosis (OR 10.04, p = 0.018). It was unexpected that a lower AJCC02 stage was an independent predictor of mortality, this finding could be related to tumor biology however no death cell markers were performed in the biopsy samples.
Variables with independent prognostic value on the multivariate analysis.
| Variable | OR | p value |
|---|---|---|
| Treatment | 19.57 | 0.001 |
| No treatment | 13.83 | 0.000 |
| RFA | 3.82 | 0.05 |
| Tumor Differentiation | 10.04 | 0.018 |
| Moderate | 6.49 | 0.010 |
| Poor | 6.51 | 0.011 |
| AJCC02 | 6.58 | 0.15 |
| Stage 2 | 4.82 | 0.02 |
| Stage 4 | 2.19 | 0.13 |
OR: Odds Rate. RFA: Radiofrequency ablation.
Epidemiological trends demonstrate that HCC has become one of the 20 causes of death among general population in Mexico.5 Latin-American countries are considered a HCC moderately high risk area compared to other parts of the world, with incidence rates similar to those of Italy and Spain (11 to 20 cases x 100,000 habitants).3 This increase in HCC suggests an increase in the awareness of hepatic disease as well as the result of the natural history of liver disease. Cirrhosis of any cause and other hepatic conditions such as non-alcoholic steatohepatitis and viral hepatitis are known risk factors for the development of HCC.15,16 In our study we found an increase referral on HCC to our hospital. This increase is evident as the average rate of referral to medical centers where HCC treatment opportunities has risen slightly. It is important to emphasize that the studies reported on Mexico’s HCC rate have collected patients in ten years span while our study comprehends four years of consecutive patients that may point out a slight increase on the referral associated to a higher frequency of this disease.8,9
There is a poor understanding of the risk factors associated to HCC and therefore the results we had obtained in treating it has been limited since patients were sent in advanced stage. Although cirrhosis is the main risk factor associated to HCC, some other conditions are associated as mentioned earlier. For example, we found 27.7% patients without cirrhosis; over half of them diagnosed with diabetes mellitus that is a recognized HCC risk factor, associated to a 3.4 fold HCC risk increase.16 As in most HCC series, our report shows viral hepatitis C is a major HCC risk factor. Recently, a shift in the etiology of cirrhosis in Mexico has been described that reveals that viral hepatitis is as important as alcohol intake.17
In Mexico, the medical community is not fully aware of HCC surveillance in high-risk patients. In addition, treatment options are not completely recognized. A pitfall to these circumstances is that HCC is diagnosed in advanced stage where curative treatments are not feasible. As it is shown in table 1, AJCC02 staging 3 represented 55.3% of cases and 21.3% were stage 4. BCLC staging showed advance disease as well since 51.1% of patients were on D stage, suggesting null curative options and few choices of palliative treatments. This is in concordance with previous reports in Mexico, as in the study of Mondragon where it was found that tumor median size was 8 cm, same as our series, nevertheless he did not describe staging in any classification system.8 On the other hand, Meza and Candelaria study informs AJCC stage 3 and 4 in 74%,9 extremely similar to our series where 76.6% consist of stage 3 and 4. We found patients age to be 60.2 years old that is comparable in Mexican and international reports.7,8,13,15,16 In the univariate analysis from clinical and biochemical variables that were associated to the hepatic disease, only the absence of ascites and a normal serum creatinine value were correlated to survival, although, in the multivariate analysis were not significant. Child-Pugh classification was similar to previous reports in Mexican population,7 as the majority of patients Child-Pugh class are B, this in contrast to Mondragon report that informs the largest part in Child-Pugh C class.8 Child-Pugh was closely associated to survival in univariate analysis but not in multivariate analysis. This is not surprising, as it has been reported in other studies, where performance status and vascular invasion are prognostic factors.13,18 It seems that HCC survival is not dependent only on liver function but also in the spread of disease. This suggest that controlling spread can be part in palliating disease, while better treatment becomes available to improve survival of this deadly disease. A disadvantage of this study is that we could not verify the precise cause of death, since many patients died outside Mexico City.
In the last decade there has been an advance in treatment options. BCLC System has been adopted since offers the advantage to guide in treatment options.19-21 A good patient selection allows a better survival; this has been evaluated by several groups that have obtained three years survival over 50%. Another feature that allows a better survival is the improvements on the day to day care of patients with liver disease, such as prevention of variceal bleeding, prevention of infections, nutritional support, complications screening and patient education. In addition, new chemotherapy agents have become available that advanced disease patients may benefit from. Traditionally, HCC treatment options have been classified as curative or palliative. Surgery and liver transplantation are understood to be curative, while RFA, TACE and chemotherapy are considered palliative. In our study, surgery and TACE have similar survival rates. It is remarkable that in the Mexican population, ablative treatment procedures such as ethanol injection or RFA, had a lower survival than TACE, since patients exposed to TACE have larger tumors and are in a more advance stage.9 From these results, it appears that, in our country, TACE is a good palliative treatment option. However many patients have liver-disease coagulopathy that makes interventional procedures risky, as bleeding complicates outcomes. Conventional chemotherapy based in doxorubicin had demonstrated modest palliation rates but it has been prescribed to patients with preserved liver function (Child-Pugh A) due to adverse effects that can compromise blood cell counts.22,23 New agents such as Sorafenib (Nexavar®), are promising drugs added to HCC treatment, though there is still the problem of prescribe it to patients with moderate or advance liver disease (Child-Pugh B) as more adverse effects can be expected.24 HCC is a disease that can benefit from interdisciplinary approach, including specialists on oncology, gastroenterology-hepatology, interventional radiology and liver transplantation, which offers the best diagnostic and therapeutic options while controlling side-effects. It is important to point out that those patients that have any treatment have a better survival than patients that did not have any option, regardless of liver function.
In conclusion, this study shows a slight but significant increase in the referral of HCC to a medical center that suggests is related to the increase of HCC in Mexico. Viral hepatitis C is the main risk factor associated to HCC, followed by Diabetes mellitus and Alcohol-associated liver disease. Early stages on AJCC and BCLC classification systems correlated with survival. However, due to advance disease staging diagnosis, TACE has been the best treatment option. As new agents become available, it is possible to improve survival on a traditionally deadly disease.