Introduction-Aim. Health-Related Quality of Life (HRQOL) has become an important focus of patient care and clinical outcomes research with the improvement in patient and graft survival after liver transplantation (LT). The current study was designed to evaluate the post-transplant HRQOL profiles using the Liver Disease Quality of Life 1.0 (LDQOL 1.0) Questionnaire and demonstrate the possible effect of peri-transplant clinical covariates on these profiles.
Material and methods. Participants included pre-transplant group (waiting-list patients n = 50) and post-transplant group (mean 5 ± 4 years after deceased or living donor LT n = 103) who were recruited from 3 specialized centers in Egypt. We applied the LDQOL 1.0 questionnaire; a 111-item containing the Short Form-36 version 2.0 (SF-36v2) as a generic component supplemented by 75 disease-specific items. The etiology of cirrhosis, co-morbidities, model for end-stage liver disease (MELD), Child-Pugh class and post-operative complications were analyzed.
Results. All recipients had significant higher HRQOL scores than patients in waiting-list using both questionnaire components. Recipients with pre-LT MELD ≥ 15, Child-Pugh class C, history of hepatocellular carcinoma (HCC) demonstrated low HRQOL scores. Recipients without post-operative surgical complications had a statistically better HRQOL using the disease-specific, but not the SF-36v2 component. On the other hand, both components demonstrated non-significant lower scores in recipients with rejection episodes, cytomegalovirus (CMV) infection and hepatitis C recurrence had compared to those without medical complications.
Conclusion. Generally HRQOL improves dramatically after LT as assessed by LDQOL questionnaire. Moreover, combined questionnaires can provide accurate information about the possible impaired HRQOL post-LT due to pre-transplant disease severity and post-operative complications.
Patient and graft survival rates have improved dramatically since the inception of the liver transplantation life-saving procedure. With the achievement of 5-year, 10-year patient and graft survival at 70, 60 and 67% respectively,1,2 attention has focused on improving quality of life in transplant recipients. The overall improvement of health-related quality of life (HRQOL) could occur during the first year posttransplant and may be sustained; however, the overail quality of life will be still less relative to the general population.3 The collective influence of peritransplant clinical events on HRQOL outcomes has not been well described. Patients’ gender, primary diagnosis, hepatic decompensation,4 personality,5 in addition to the disease recurrence, post-operative complications and the onset of de novo diseases were postulated to impair the HRQOL. Improved quality of life included cognitive, mental, physical and social functioning,6 however, other specific aspects may vary among transplant recipients.
Health-related quality of life research presents a challenging goal for clinicians as it enables them to recognize how clinical events may affect the HRQOL profiles and convert information based on patients’ reports into quantitative measurements that must be properly selected and standardized. These measurements can be divided into disease-specific and generic assessment. Disease-specific instruments for liver diseases include the liver disease quality of life7 chronic liver disease questionnaire (CLDQ),8 and hepatitis quality of life questionnaire9 while the Medical Outcome Short Form-36 (SF-36) is a generic instrument.10 The liver disease quality of life (LD-QOL1.0) instrument uses the Short Form-36 version 2.0, supplemented by 75 disease-targeted items grouped into 12 domains that can evaluate symptoms related to hepatic disease and the patient’s perception about the stigma of liver disease.7
A specific understanding of all HRQOL profiles and their predictors in LT recipients represent a main confront that needs to be elucidated in Egypt. Thus the identification of such variables will allow a more comprehensive evaluation and complement survival predictions with ultimate improvements in quality of life.
AimThe current study was designed to evaluate the post-transplant HRQOL profiles in liver transplant recipient using the Liver Disease Quality of Life 1.0 (LDQOL 1.0) Questionnaire and demonstrate the possible effect of pre and post-transplant clinical covariates on these profiles.
Material and MethodsStudy populationThis prospective study was conducted on a cohort of 153 adult ambulatory patients attending regular follow-up at Agouza Police Hospital, Agouza, Dar-Al Fouad Hospital; 6th October City and Kasr Al-Aini Hospital, Cairo University, Cairo, Egypt from 2007-2009. All participants were invited to participate in the current study after explaining the translated Arabic version of health-related quality-of-life (HRQOL) survey. Both generic; SF-36 and disease specific; LDQOL questionnaire components was explained, redundant questions were removed and the participation was strictly voluntary. An informed consent was obtained from all participants and the study was approved by the institutional ethical committee which involves the administration of this questionnaire and its integration with patients’ data.
Inclusion criteriaAdult, fully-conscious, ambulatory, LT-recipients and candidates who were able to complete the questionnaire with no other co-morbid cardiac, pulmonary, renal diseases.
Liver transplant recipients; after either deceaseddonor liver transplantation (DDLT) done at a period ranging from 1-10 years or living-donor liver transplantation (LDLT) done at a period ranging from 1-5 years were recruited from Agouza Police Hospital, Kasr Al-Aini Hospital and Dar Al-Fouad Hospital. The QOL survey was obtained only once to all participants after a mean post-transplant follow-up period of 4 ± 5 years in either transplant sub-groups. At the same time, patients with end stage liver disease (ESLD) who were listed for LT were considered as the control group to overcome the fact that the survey was not conducted pre-LT.
We could not determine the actual time of HCV acquisition and thus a range of years of suffering with liver disease in either LT population or ESLD was not determined. Given that there was no significant difference as regards the age of both groups, it can be assumed that both groups have acquired HCV infection during their early life as they have history of parenteral anti-bilharzial therapy. Moreover both Child-Pugh and MELD scores did not show any significance which indicated that they had a comparable disease severity.
Both LT and ESLD groups were educated (ranging from high school-university graduate); and some of the LT patients were supported by health insurance related to their work, but not by a special private insurance.
Exclusion criteriaPatients with fulminant hepatic failure, candidate for multiple-organ transplantation and patients refusing to participate or cannot complete the survey.
Data collectionPre-transplant demographic and clinical measures included age, sex, primary diagnosis, Child and Model of End-Stage Liver Disease (MELD) score.
Post-transplant clinical outcomes that were hypothesized to have an effect on HRQOL were surgical complications, disease recurrence, cytomegalovirus infection or rejection episode that occurred prior to the survey.
Clinical HCV recurrence post-LT was diagnosed by the evidence of viral replication by HCV-PCR, elevated transaminases and confirmatory histology11 which was clearly differentiated from pathologic allograft rejection.
Moreover, infectious episode was defined as a positive blood or urine culture for bacterial, fungal, or viral pathogens that required treatment. Cytomegalovirus (CMV) infection was defined as CMV infection requiring anti-CMV therapy.
Survey instrumentsLiver transplant recipients as well as the control group were subjected to a 111-item self-report measure-LDQOL 1.0 questionnaire-containing the Medical Outcome Short Form-36 (SF-36) health survey version 2.0 as the generic core supplemented by the disease-targeted component; LDQOL.7
The SF-36 is a well-validated generic questionnaire that can evaluate physical and mental health.10 It includes 36 items divided into eight domains: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH).
Scores obtained for each scale were aggregated into a mental and physical component summary (MCS) and (PCS). Scale scores range between 0 and 100; higher score indicates less impairment whereas a lower score suggests greater impairment.
LDQOL is a disease specific questionnaire that includes 12 disease-specific domains containing 75 items which can measure the impact of chronic liver disease on functioning and well-being. The LDQOL domains are:7
- •
Symptoms of liver disease (17 items).
- •
Effects of liver disease (10 items).
- •
Concentration (7 items).
- •
Memory (6 items).
- •
Quality of social interaction (5 items).
- •
Health distress (4 items).
- •
Sleep problems (5 items).
- •
Loneliness (5 items).
- •
Hopelessness (4 items).
- •
Stigma of liver disease (6 items).
- •
Sexual functioning (3 items).
- •
Sexual problems (3 items).
Qualitative variables are presented by number and percent. Different groups were compared by Chi-square test or Fischer’s exact test when appropriate. Quantitative variables were presented by mean and standard deviation (SD). Comparison between two groups considered significant if < 0.05.
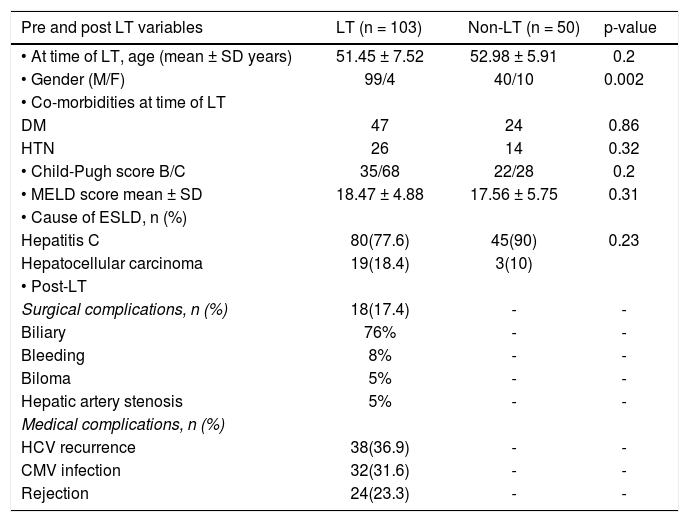
ResultsThe characteristic features of LT population and non-LT population is demonstrated in table 1. There was male predominance and mean age was within the 6th decade of life among both studied groups with no significant difference. The most common indications for LT and causes for end stage liver disease were HCV-related end stage liver disease (81.6%), hepatocellular carcinoma (13.7%), in addition to HBV, and cryptogenic cirrhosis. A rather similar high percentage of HCV-related ESLD was observed among the non-LT population. Severity of liver diseases, as assessed with Child-Pugh and model of end-stage liver disease (MELD) scores, showed no statistically significant difference among both population, p value 0.2 and 0.3 respectively. The mean time post-transplant was 5 ± 4 years (range 1-9 years), 17.4% had surgical complications, 36% had HCV recurrence, 31% had CMV infection and 23% experienced one or more episode of rejection post-transplant (Table 1).
Characteristic features of both liver transplantation and non liver transplantation population.
| Pre and post LT variables | LT (n = 103) | Non-LT (n = 50) | p-value |
|---|---|---|---|
| • At time of LT, age (mean ± SD years) | 51.45 ± 7.52 | 52.98 ± 5.91 | 0.2 |
| • Gender (M/F) | 99/4 | 40/10 | 0.002 |
| • Co-morbidities at time of LT | |||
| DM | 47 | 24 | 0.86 |
| HTN | 26 | 14 | 0.32 |
| • Child-Pugh score B/C | 35/68 | 22/28 | 0.2 |
| • MELD score mean ± SD | 18.47 ± 4.88 | 17.56 ± 5.75 | 0.31 |
| • Cause of ESLD, n (%) | |||
| Hepatitis C | 80(77.6) | 45(90) | 0.23 |
| Hepatocellular carcinoma | 19(18.4) | 3(10) | |
| • Post-LT | |||
| Surgical complications, n (%) | 18(17.4) | - | - |
| Biliary | 76% | - | - |
| Bleeding | 8% | - | - |
| Biloma | 5% | - | - |
| Hepatic artery stenosis | 5% | - | - |
| Medical complications, n (%) | |||
| HCV recurrence | 38(36.9) | - | - |
| CMV infection | 32(31.6) | - | - |
| Rejection | 24(23.3) | - | - |
LT: liver transplantation population. Non-LT: not liver transplantation population. ESLD: end-stage liver disease. DM: diabetes mellitus. HTN: hypertension. p-value < 0.05 is considered significant.
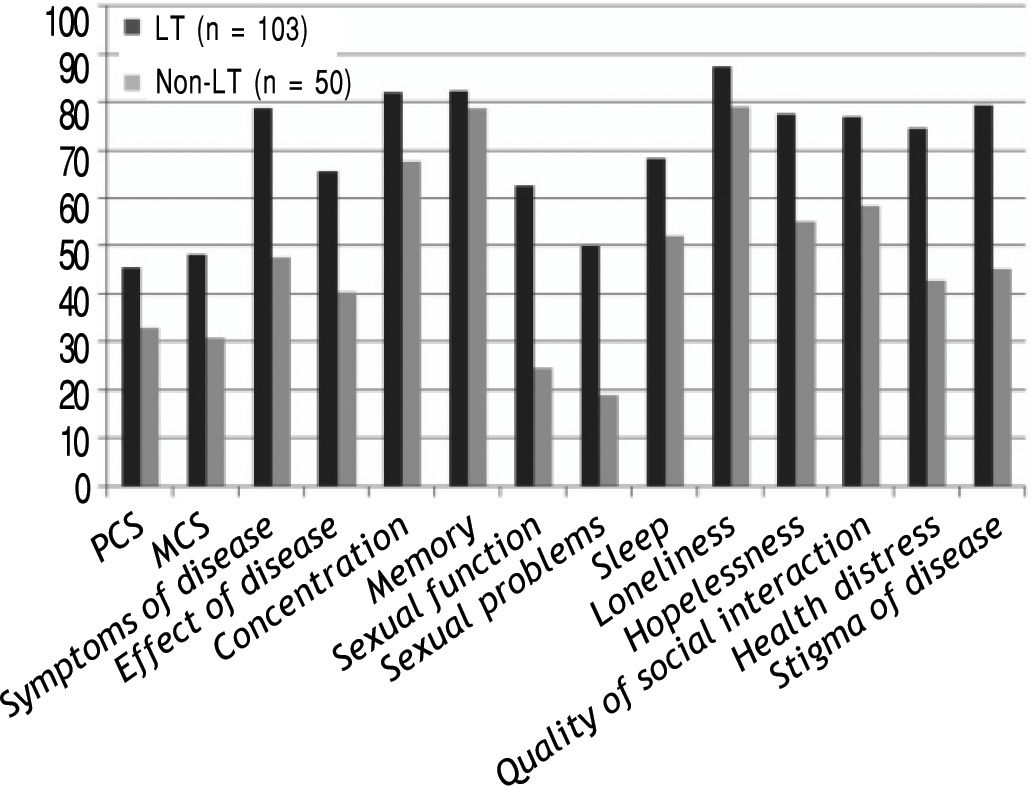
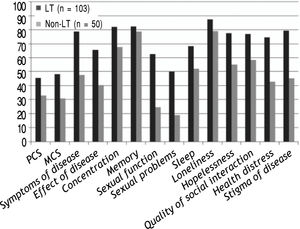
HRQOL scores of both generic; SF-36 and 11/12 (91.6%) of the disease-specific domains were significantly higher in LT cohort (n = 103) compared with the patients on the transplant list, p < 0.01 as illustrated in figure 1.
Post-transplant quality-of-life domains were associated with several pre and post-transplant factors such as MELD, Child class, HCC, surgical and medical post-operative complications. HRQOL scores were evaluated in LT population in relation to the pre-operative assessment of liver disease severity according to the MELD scores and the different classes (A-C) of the Child-Pugh classification.
Better quality of life was displayed among LT population in all 12 domains of the LDQOL and both main components of generic SF-36 questionnaire, but with no significant difference among patients with MELD score < 15, whereas, only 5/12 (42%) of the disease-specific domains and PCS of SF-36 showed better HRQOL in patients with Child B with statistically significant for memory only (P = 0.03).
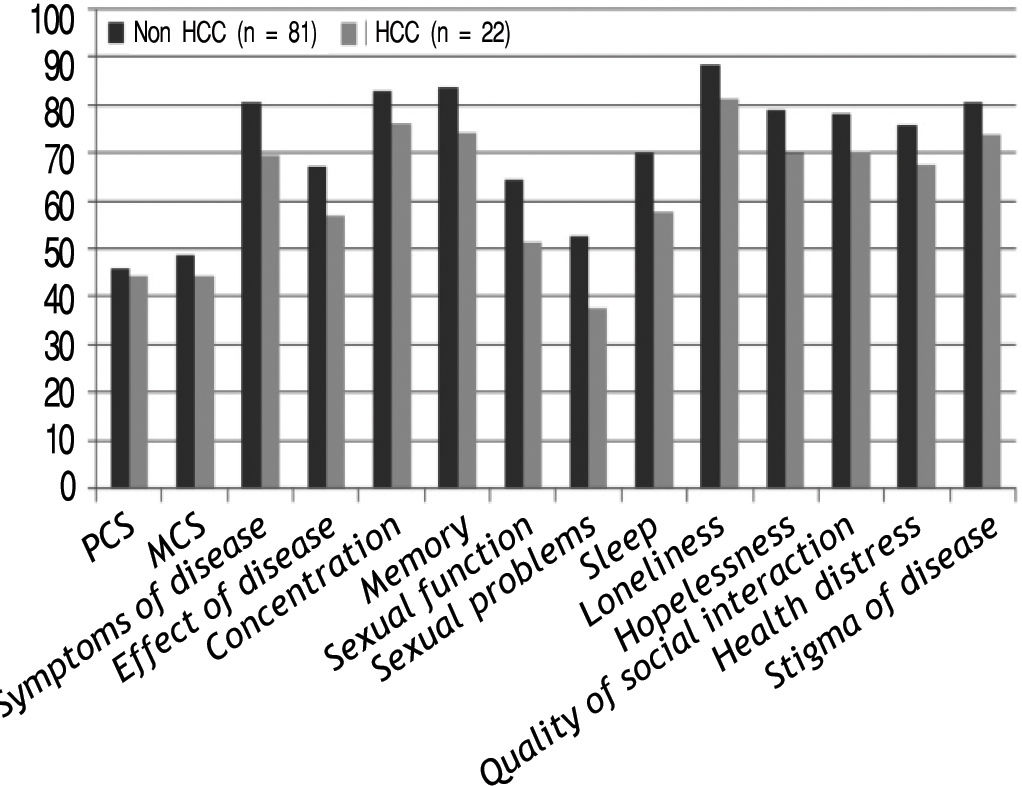
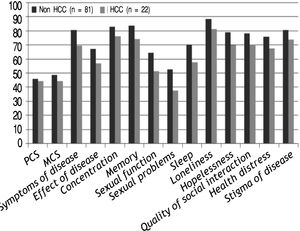
Patients with pre-LT HCC demonstrated lower scores for both components compared to those with no HCC with significant difference for 6/12(50%) of the disease-specific domains as shown in Figure 2.
Post-operative surgical complications occurred in 17.4% of transplant population, 12 % and 22.6% in DDLT and LDLT respectively. On the other hand, medical complications such as rejection and CMV infection were significantly higher in LDLT group compared to DDLT, p value 0.002 and < 0.01 respectively, however, HCV recurrence did not exhibit such significant difference.
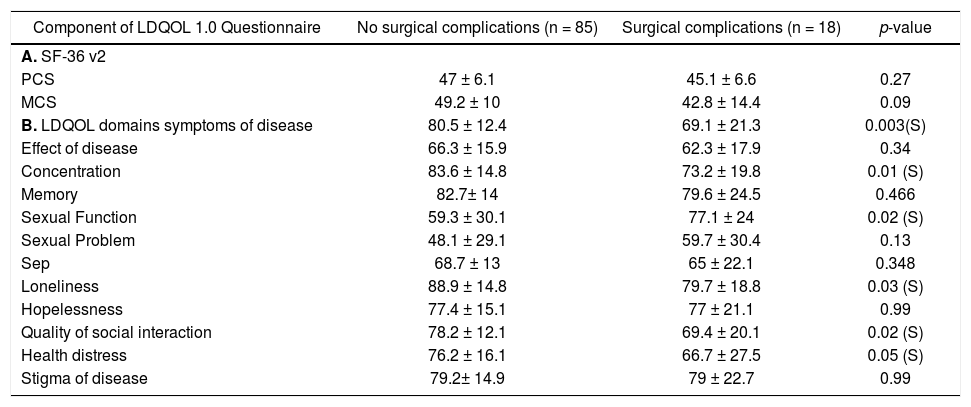
The quality of life domains were analyzed in relation to the post-LT complications. Patients with surgical and medical complications demonstrated lower scores for both generic and disease specific components. A significant difference was noted for 6(50%) of the 12 domains of the disease specific component in relation to the presence of surgical complications as shown in table 2, however, such significance was not evident in relation to medical complications. The presence of CMV infection, episodes of cellular rejection or HCV recurrence demonstrated significant scores of the quality of life domains in 25% (3 domains), 16% (2 domains) and 8% (one domain) respectively.
Components of LDQOL 1.0 Questionnaire in relation to the post-LT surgical complications among transplant population.
| Component of LDQOL 1.0 Questionnaire | No surgical complications (n = 85) | Surgical complications (n = 18) | p-value |
|---|---|---|---|
| A. SF-36 v2 | |||
| PCS | 47 ± 6.1 | 45.1 ± 6.6 | 0.27 |
| MCS | 49.2 ± 10 | 42.8 ± 14.4 | 0.09 |
| B. LDQOL domains symptoms of disease | 80.5 ± 12.4 | 69.1 ± 21.3 | 0.003(S) |
| Effect of disease | 66.3 ± 15.9 | 62.3 ± 17.9 | 0.34 |
| Concentration | 83.6 ± 14.8 | 73.2 ± 19.8 | 0.01 (S) |
| Memory | 82.7± 14 | 79.6 ± 24.5 | 0.466 |
| Sexual Function | 59.3 ± 30.1 | 77.1 ± 24 | 0.02 (S) |
| Sexual Problem | 48.1 ± 29.1 | 59.7 ± 30.4 | 0.13 |
| Sep | 68.7 ± 13 | 65 ± 22.1 | 0.348 |
| Loneliness | 88.9 ± 14.8 | 79.7 ± 18.8 | 0.03 (S) |
| Hopelessness | 77.4 ± 15.1 | 77 ± 21.1 | 0.99 |
| Quality of social interaction | 78.2 ± 12.1 | 69.4 ± 20.1 | 0.02 (S) |
| Health distress | 76.2 ± 16.1 | 66.7 ± 27.5 | 0.05 (S) |
| Stigma of disease | 79.2± 14.9 | 79 ± 22.7 | 0.99 |
MCS: mental component summary. PCS: physical component summary. p-value < 0.05 is considered significant.
Health Related Quality of Life (HRQOL) evaluation has been integrated into the liver transplantation outcomes.12,13 The major concern is determining whether the HRQOL has improved with respect to the situation prior to the procedure rather than only post-LT. Moreover, HRQOL measurements are mostly dependent on patient self-assessment, experiences, beliefs, and perceptions. Thus the choice of an HRQOL instruments depends on the purpose of the study and the intended population14 that can translate subjective patient experience into quantitative measurements.15
The present study was designed to evaluate the post-LT HRQOL profile in a cohort of 103 patients who have underwent either DDLT or LDLT using the Liver Disease Quality of Life 1.0 (LDQOL 1.0) questionnaire and the different peri-transplant parameters which can influence that profile.
Our analysis was able to demonstrate better quality of life post-LT as assessed by both disease-specific and generic components of the LDQOL questionnaire compared to the non-LT population who represented the potential scores in the pre-LT period. This was evident by the significant high scores for both physical, mental components summary SF-36 v2 in addition to 91% of the disease specific questionnaire domains in all LT patients; DDLT and LDLT. The overall improvement in mental and physical HRQOL in addition to the hepatic-related symptoms has been well established by the administration of a combined disease-specific and generic questionnaire in the transplantation setting. A rather similar study that applied both generic (the SF-36) and a liver disease specific instrument; Chronic Liver Disease Questionnaire (CLDQ) to LT recipients could specify the effects of socioeconomic and demographic differences on the HRQOL outcomes. However, it is unclear whether these instruments can assess any consequences which are exclusive to the transplant population such as immunosuppressive therapy, rejection, and disease recurrence.16
In accordance to our findings, the use of disease-targeted instruments have previously expressed better HRQOL as demonstrated by improvements in a patient’s self-image and improved perception of status post-LT.17,18 Alternatively, the generic SF-36 questionnaire could illustrate the severe impairment of HRQOL better than the domain-specific instruments19 characteristically among patients awaiting LT,20 with ESLD21 in addition to the differences in QOL before and after LT.22,23 On the other hand, the SF-36 may neglect some specific aspects,22,23 the adverse effects of immunosuppressive drugs, rejection, the lack validation of changes in patient responses over time cannot be expressed with the use of generic HRQOL instrument alone.12
The characteristic features of our study population expressed a significant male predominance, mean age in the early fifties and high percentage of HCV as an indication for LT. The reported age group reflects the tendency of patients to seek medical advice at old ages owing to the slow progression and the rather few symptoms of HCV-related liver disease. This may indicate that most of patients had acquired HCV infection earlier during their active phases of life. Moreover, the significant male predominance could suggest the likelihood of high exposure to HCV and the relative rapid progression of hepatic fibrosis with the eventual need for LT.24 In the current study, 77% of patients underwent LT secondary to HCV followed by HCC owing to the high prevalence of HCV-chronic liver diseases and its consequences in Egypt. A rather similar finding; 88.7% and 89.8% were previously reported in Egypt as well respectively.25,26 Similar to the LT population, the ESLD patients (non-LT population) exhibited a high percentage of HCV-related end stage liver disease compared to HCC patients. Thus both non-LT and LT population from the 3 transplant centers were comparable as regards the burden between liver disease and HCC.
Furthermore, recipients with history of HCC had lower HRQOL scores compared to those with no HCC with significant results for 60% of the disease-specific domains. Although HCC usually resolves with liver transplantation, however, the fear of possible recurrence of this malignancy and its consequences may have a negative effect on post-LT QOL scores. In consistent with our results, pre-trans-plant HCC was significantly associated with post-transplant quality of life.27
The model for end stage liver disease(MELD) score; a liver-disease scoring index; has replaced the Child-Turcott Pugh score for allocation of organs in patients with ESLD awaiting LT in addition to its role as an overall indicator of the patient’s health status. The present study was able to demonstrate better HRQOL scores in recipients with Child-B class and lower MELD scores compared to these with Child-C and MELD score > 15. Similarly, recipients with higher pre-LT MELD scores as well as Child-Pugh class C had worse HRQOL28 and an increase in MELD was negatively correlated with physical functioning domains of the SF-36 questionnaire.20 In contrast, high pre-transplant MELD score, > 18, was found to have a positive effect on the physical components which may be due to self-perceived improvement in HRQOL in comparison to their pre-operative status,15 but not mental HRQOL.29
On the other hand, the application of a different HRQOL assessment, the Chronic Liver Disease Questionnaire, was not able to identify any significant impairment in HRQOL according to MELD scores27 in contrast to Child-Pugh scores.
The different scales used in the respective HRQOL questionnaires may explain any divergent results related to the influence of either MELD or Child-Pugh class. Impaired mental dimensions due to the presence of hepatic encephalopathy30 while sexual functioning probably reflects the effects of liver disease.31 Moreover, lower memory scores,28 greater health distress and loneliness were statically significant with higher MELD scores and Child-Pugh class C.32 On the other hand, the symptoms of liver disease and effects of disease domains were significantly related to the Child-Pugh classification, but not to the MELD scores. This emphasizes the importance of the former in cirrhotic patients, as there is no consensus that its performance is inferior to that of MELD scores.33
The postoperative outcome varies greatly depending on the patient’s preoperative state, the quality of the donated organ, and the complexity of the surgery.34 In the present study, biliary complications occurred among 17.4% of recipients and were more evident in LDLT. Medical complications; rejection and CMV infection; were significantly reported among LDLT group compared to DDLT, p value 0.002 and < 0.01 respectively with no significant difference for HCV recurrence. Previous studies had addressed biliary complications particularly in the setting of LDLT.35 The considerable lower HRQOL scores among recipients with post-LT complications as expressed in more than 80% of the disease-specific domains. This impairment might be related to the various procedures, either interventional radiology and/or endoscopy or even surgical intervention to reach a definitive resolution.35
In the present series, 23% of recipients who had experienced one or more histologically-confirmed episode of rejection expressed impaired HRQOL scores with significant changes in 16% of disease-specific domains. These impairments could be explained by the additional procedures, hospital admissions, frequent outpatient visits subjected to the recipients secondary to these episodes. Similar rate of cellular rejection was noted in patients who were subjected to either change in their immunosuppression both with the subsequent significant lower scores on SF-36.15 Consistent rates of cellular rejection were reported in previous studies with comparable immunosuppression regimens.36,37
The high prevalence of HCV in Egypt with the ultimate universal disease recurrence post-LT has highlighted the pronounced effect of recurrent diseases on HRQOL scores. Recipients with HCV demonstrate lower scores than those transplanted for HBV or alcoholic cirrhosis.38 Thus it was very important to address the effect of HCV recurrence (36% of LT-population) by the LDQOL questionnaire which demonstrated non-significant lower HRQOL scores post-LT. These scores may reflect the sense of living with an impending threat in addition to the greater contributor to depression. Indeed, the low HRQOL scores in recurrent HCV was related to recipients’ knowledge of their own viral status rather than the timing of any physical consequences of recurrent liver disease or any related medical complications.39
In consistence to our results, HRQOL improved significantly post-LT without significance difference with respect to the HCV recurrence at 6 months post-LT, with significant lower QOL, and greater depressive symptoms at one year.40 Likewise, both SF-36 components’ scores were lower due to the psychological stress concerned with disease recurrence.41-43 Moreover, the disease-specific and not the SF-36 questionnaire could exhibit significant worse HRQOL post-LT with HCV recurrence.44 This may be due to the effect of the presence of the virus itself or the possibility of anti-viral therapy45 which was not present in our series.
In the present series, recipients with CMV infection (31%) had lower HRQOL scores compared to those with no infection with significant scores in only 25% of disease-specific domains. The slightly impaired QOL might be related to the sense of illness or frequent investigations done to diagnose such infection which tends to be in need of a laborious work-up due to the wide differential diagnosis and the attenuation of clinical manifestations because of the immunosuppressive medication.
To the best of our knowledge, HRQOL and the possible impact of peri-transplant factors were not previously assessed among the Egyptian transplant population. However, our analysis was constrained by several limitations. The absence of a routinely used transplant-specific HRQOL instrument, the fact that it was not done pre-LT, the exclusion of the patients in poor clinical condition and not considering the effect of cultural, economical and social factors in addition to the lack of other etiologies rather than HCV-related. Moreover, this questionnaire is rather time-consuming and needs a good physician-patient relationship to ensure that accurate answers are provided.46
ConclusionsSuccessful liver transplantation ought to consider the HRQOL measures in addition to the substantial survival rates. The application of both generic and disease-specific questionnaires might provide greater specificity and sensitivity for patients’ outcome.
Abbreviations- •
CMV: cytomegalovirus.
- •
DDLT: deceased-donor liver transplantation.
- •
ESLD: end-stage liver disease.
- •
HCC: hepatocellular carcinoma.
- •
HRQOL: Health Related Quality of Life.
- •
LDLT: living-donor liver transplantation.
- •
LT: liver transplantation.
- •
MCS: mental component summary.
- •
MELD: model for end-stage liver disease.
- •
PCS: physical component summary.
- •
SF-36: short form-36.
I hereby declare that there are no financial or non financial competing interests for any of the authors.