Background. Patients with chronic HCV infection and superinfection by hepatitis A virus (HAV) or hepatitis B virus (HBV) have higher morbidity and mortality when compared with those without HCV infection. Therefore, HAV and HBV active immunization has become mandatory in this population and hence their serological markers must be determined. The aim of this study was to evaluate the prevalence of serological markers of HAV and HBV infection in patients with chronic HCV.
Material and methods. One thousand chronic HCV patients at the University of Säo Paulo School of Medicine were evaluated for the prevalence of serological markers of HAV and HBV infection.
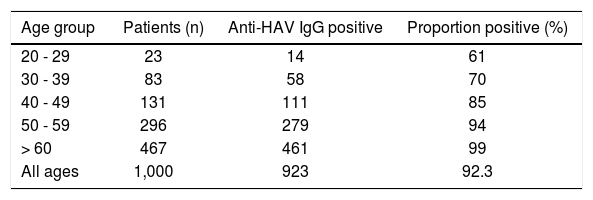
Results. Anti-HAV IgG was positive in 92.3% of patients. When stratified by age, anti-HAV IgG was found in 61% of patients between 20-29 years, 70% on patients between 30-39 years, 85% on patients between 40-49 years, 94% on patients between 50-59 years, and in 99% on patients over 60 years of age. Anti-HBc IgG was positive in 244 patients (24%). Stratified by age, in 4.3% of patients between 20-29 years, 17% 30-39 years, 21% 40-49 years, 24% 50-59 years, and in 28% of patients over 60 years. Of the 244 anti-HBc IgG positive patients, 0.8% were HBsAg positive, 8.5% were anti-HBc IgG isolated and 16% were also anti-HBs positive.
Conclusions. In conclusion, the prevalence of anti-HAV IgG was similar to the general Brazilian population. However, anti-HBc IgG was higher in our patients, when compared to general population of Western countries, emphasizing the importance of immunization programs for this population.
Hepatitis C virus (HCV) is a leading cause of chronic liver disease, cirrhosis, and hepatocellular carcinoma (HCC), as well as the most common indication for liver transplantation in Western countries,1,2 About 150 million people are chronically infected with HCV, and more than 350 000 individuals die every year from hepatitis C-related liver diseases.3
Acute hepatitis A virus (HAV) infection usually presents itself as a self-limiting infection with fatality rates varying from 0.01% to 0.5% in adults.4 However, HAV superinfection in patients with chronic liver disease, including patients with chronic hepatitis caused by HCV is associated with a high risk of fulminant hepatitis and consequently with death.5-10 The most dramatic observation of HAV superinfection in patients with chronic HCV infection has been demonstrated in an Italian study.10 This study followed 432 patients with chronic hepatitis C (183 with cirrhosis) for a period of 7 years. All patients underwent liver biopsies one year prior to study initiation and were tested every 4 months for the detection of antibodies to HAV. Seventeen patients presented HAV superinfection during the study. Among these patients, seven cases progressed with fulminant hepatitis with death occurring in six of them.
Several studies show that patients with chronic HCV infection and co-infection with hepatitis B virus (HBV) have an accelerated progression to liver fibrosis and cirrhosis and carry a higher risk of HCC when compared with patients with chronic HCV infection alone.11-19 Thus, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend that patients with chronic liver disease, including those with chronic hepatitis C, should be vaccinated against both HAV and HBV.7,20–22
Vaccination against HAV or HBV shows low efficacy when administered to patients with advanced liver disease, particularly in decompensated patients awaiting liver transplantation or in post-transplanted immunossupressed patients.13–14 However, testing for antibodies against HAV or HBV and vaccination for both viruses in patients with chronic liver diseases is still not universal in clinical practice.23,24
It is of paramount importance to evaluate the serological profile of hepatitis A and B in patients with chronic HCV infection and subsequently to refer those with negative se-rology for one or both viruses to specific immunization. So, the aim of this study was to evaluate the prevalence of serological markers of HAV and HBV infection in a specific population of patients with chronic hepatitis C.
Material and MethodsWe considered a prospective/retrospective study of serological markers of previous contact with hepatitis A (anti-HAV IgG - Abbott-Axsym - MEIA. Chicago, USA) and past or current hepatitis B: anti-HBc IgG (Abbott Architect electrochemiluminescence - CMIA. Chicago, USA), HBsAg (Abbott - Architect electrochemiluminescence - CMIA. Chicago USA) and anti-HBs (Abbott -Axsym - MEIA. Chicago, USA) in 1.000 adult chronic HCV infected patients of both genders followed at the outpatient Liver Clinic at the University of São Paulo School of Medicine Hospital. Chronic HCV infection was determined by the presence of anti-HCV (VITROS anti-HCV assay, Ortho-Clinical Diagnostics Inc. Buckinghamshire, UK) and HCV-RNA (Abbott Real time HCV PCR, Chicago, USA) for more than 6 months. The patients were assessed either retrospectively or prospectively from March 2010 to Dec 2013. We accepted serology obtained up to 6 months before inclusion in the study. In patients with HAV or HBV serological markers older than 6 months, we performed new tests.
The serological data were collected either from medical records or from laboratory data bases. Patients who did not have previous markers for HAV and/or HBV were subsequently evaluated after work up. Patients who tested negative for hepatitis A (anti-HAV IgG) and/or hepatitis B (anti-HBc IgG) markers were referred for immunization. All serological tests and PCR were performed at the central laboratory at the University of Sāo Paulo School of Medicine Hospital. The samples were collected and processed on the same day and storage at minus 80° degrees Celsius for a week. This study was conducted in accordance to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institutional Internal Review Board.
Statistical analysisPrevalence of serological markers for hepatitis A (anti-HAV IgG) and hepatitis B (anti-HBc IgG, anti-HBs and HBsAg) was computed and the results were also expressed by age groups.
ResultsWe evaluated 1,000 consecutive chronic HCV patients. Five hundred fourteen patients were male (51.4%), and 486 were female (48.6%).
Prevalence of serologic marker anti-HAV IgG in patients with chronic HCV infectionAmong the 1,000 evaluated patients, anti-HAV IgG was positive in 923 of these patients (92.3%). When patients were stratified by age, the anti-HAV IgG was found in 14 (61%) patients between 20 and 29 years, in 58 (70%) patients between 30 and 39 years, in 111 (85%) patients between 40 and 49 years, in 279 (94%) patients between 50 and 59 years, and in 461 (99%) patients over 60 years of age. Age specific prevalences are presented in table 1.
Prevalence of anti-HBc IgG in patients with chronic HCV infectionAmong the 1,000 evaluated patients, anti-HBc IgG was positive in 244 (24%). When patients were stratified by age, anti-HBc IgG was found in 1 (4.3%) patient between 20 and 29 years, in 14 (17%) patients between 30 and 39 years, in 27 (21%) patients between 40 and 49 years, in 70 (24%) patients between 50 and 59 years, and in 132 (28%) patients over 60 years of age. Age specific anti-HBc IgG prevalences are displayed in table 2.
Prevalence of other serologic markers of HBV infection in anti-HBc IgG positive patientsAmong 1,000 patients referred to our tertiary care outpatient Liver Clinic from primary and secondary centers and included in our study, 42% did not have previous serological tests performed for hepatitis A (anti-HAV IgG) and 29% did not have serological results for hepatitis B (anti-HBc IgG).
Among the 244 anti-HBc IgG positive patients, 8 patients (0.8%) were also HBsAg positive, 151 (15.1%) were anti-HBc and also anti-HBs positive, demonstrating acquired immunity to HBV, and 85 (8.5%) were anti-HBc IgG isolated. Additionally, 78 anti-HBc negative patients, presented anti-HBs positive due to anti-HBV immunization.
DiscussionThe significant increase in morbidity and mortality of acute HAV and/or HBV superinfection in patients with underlying chronic hepatitis C emphasizes the importance of knowing the serological profile of these viruses in HCV infected patients and suggests referral of those patients without previous contact with one or the two viruses for immunization.10,23,25
In our study, we found a high prevalence of anti-HAV IgG, a serologic marker that indicates previous contact with HAV, among patients with chronic HCV infection. Among these patients the prevalence progressively increased with age, being higher than 90% in patients in the 50+ age group. The prevalence was lower in the younger HCV patients, suggesting that immunization should be considered for these susceptible individuals. Comparing our data with a population based study conducted in the city of São Paulo, Brazil, to assess the prevalence of HAV and HBV in the general population,26 we observed a similar distribution of anti-HAV IgG in our chronic HCV patients when stratified by age. Individuals between 50 to 59 years had a prevalence of 94% in both HCV and non-HCV populations, in the two different studies, respectively. In individuals older than 60 years of age, we have observed a prevalence of 99% in our study, comparable to 97% observed in the general population by Foccacia, et al.26
The epidemiological pattern of infection with the hepatitis A virus is changing in many developing countries. Improvements in socioeconomic conditions and hygiene practices have reduced the incidence of HAV infection, especially in individuals under 40 years of age, who are more susceptible to HAV infection. This suggests that, vaccination should be mandatory, particularly in this age group.4,6
In a retrospective study conducted in the United States (US), Sidiqui, et al. (2001) evaluated 1,092 patients with chronic hepatitis C.22 Among these patients, anti-HAV IgG tests were carried out in 671 (61%), and resulted positive in 252 (38%). Anti-HAV IgG prevalence was higher in patients above 60 years of age (76%). As hepatitis A in the US has low endemicity, the overall anti-HAV IgG prevalence in that country is lower when compared to our study with Brazilian patients. However, the prevalence of HAV infection in the US population also increases with increasing age. In another study conducted in Brazil, Devalle and colleagues (2001) evaluated the prevalence of anti-HAV IgG in 197 patients with chronic HCV infection referred to treatment.21 Anti-HAV IgG resulted positive in 170 (86%) of them. The authors attributed the high prevalence found in their study to the low socioeconomic status and to the average age above 40 years of the patients referred to their center.
In our study, we found prevalence of anti-HAV IgG positive in 85% of the patients between 40 and 49 years of age, reaching 99% in those aged 60 or more. These data demonstrate the importance of determining the serological profile of hepatitis A in patients with chronic HCV infection. It is probably the best cost-benefit strategy, compared to universal HAV immunization.
All these studies, suggest that the prevalence of HAV infection in the population of patients with chronic HCV infection is similar to that of the general population, according to the respective endemicity of HAV infection in the geographical region where the study was conducted.
The prevalence of anti-HBc IgG was positive in 244 (24.4%) of the 1,000 patients referred to our clinic. After stratification by age group, we found a higher prevalence in patients older than 30 years, increasing progressively from 17% between 30 to 39 years, up to 28% in patients older than 60 years.
The prevalence of anti-HBc IgG, a serological marker of previous contact with HBV, was higher in patients with chronic HCV infection in comparison to that of the general population of Sāo Paulo. Comparing our data stratified by age group, with a population based study,26 we observed 4.3% vs. 0.79% in the group ranging between 20 to 29 years, 17% vs. 0.54% between 30 to 39 years, 21% vs. 0.78% between 40 to 49 years, 24% vs. 4.88% between 50-59 years and 28% vs. 1.7% in patients over 60 years of age. These data suggest that patients with HBV and HCV infection share similar risk factors for acquisition of either virus. By sharing similar routes of transmission, the occurrence of coinfection or superinfection with these two viruses is not uncommon, especially in areas of high prevalence.27-32 This fact also emphasizes the importance of HBV immunization programs in this population.
In the Brazilian National Survey for the Seroprevalence of Viral Hepatitis, conducted in 2010 by Ximenes and colleagues, the prevalence of anti-HBc IgG was higher in subjects older than 20 years. This fact can be explained by a significantly increased risk of HBV exposure probably due to a more active sexual behavior in this age group.33
As recommended by the Brazilian Ministry of Health, the vaccine against HAV was incorporated into the infant immunization schedule in 2014 due to an improvement in the health conditions of the country and thus a decrease in natural contact with this virus.34 According to Brazilian guidelines, HAV and HBV immunization is also indicated otherwise for patients with chronic liver disease of any etiology, including chronic HCV. CDC and WHO also recommend that individuals with chronic liver disease, including those who had chronic hepatitis C, be vaccinated against both hepatitis A and hepatitis B.7,20–22
The absence of prior serological markers for HAV (42%) and HBV (29%) infection in chronic HCV patients referred to our tertiary care hospital by primary and secondary care centers demonstrates a problem in the management of these patients regarding proper HAV and/or HBV active immunization, as recommended by international and local guidelines.
Abbreviations- •
ACIP: Advisory Committee on Immunization Practices.
- •
CDC: Centers for Disease Control and Prevention.
- •
HAV: hepatitis A virus.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: Hepatitis C virus.
- •
WHO: World Health Organization.
We declare no conflict of interest.
Financial SupportEdvaldo Ferreira da Silva received a fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior).
AcknowledgementsE.F.S. received a fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior).