Background and rationale for the study. The generation of people born before 1965 is a high-risk group for developing chronic hepatitis C virus (HCV) infection.
Aim. To report the experience on single institution of HCV infection under birth-cohort or baby boomers effect.
Material and methods. We used a cross-sectional design of consecutive subjects older than 18 years referred for serological evaluation for anti-HCV and detection of HCV RNA.
Results. A total of 7,658 people were included. The global prevalence of HCV antibody was 4.5% (344/7658). The frequency with anti-HCV antibodies were 74 (10.9%), 158 (7.3%), and 112 (2.3%) for people born before 1945, 1945-1965, and 1966-1992, respectively (p < 0.01). The subjects HCV RNA-positive were 88.9%, 68.7%, and 44.4%, respectively (p < 0.001). The viral load was > 100,000 IU/mL in 74.4% of those positive for HCV RNA. Groups of older patients and anti-VHC, with year of birth before 1965, are more likely to show reactivity to HCV RNA and significant viral load (OR 10.0, CI 95% 4.8 to 20.1).
Conclusion. We observed a high prevalence of unrecognized chronic HCV infection. The prevalence of HCV infection in people born before 1945 was twice the value of those born after 1965. Further studies are needed to determine the impact on health care services. Future work should focus on determining the appropriate model for the care of people at risk of chronic HCV.
The hepatitis C virus (HCV) is a treatable disease, but often is an underdiagnosed condition. An estimated 130-170 million persons (~3% of the world’s population) are living with HCV infection.1 Chronic HCV infection is associated with substantial morbidity and mortality world-wide.2 In Mexico, up to 36% of chronic liver disease is related to HCV infection.3 The burden of disease in hepatitis C infection is highly significant, they are attributed to more than 350,000 deaths each year and most of these are associated with liver cirrhosis and hepatocellular carcinoma.4
In the USA, the current estimate of HCV prevalence is 1% [95% confidence interval (CI), 0.8-1.2].5 In Latin America and the Caribbean, the prevalence is 0.7% (95% CI, 0.5-1.1), which is lower than that reported for Eastern Europe, sub-Saharan Africa, and South Asia.6 Mexico is a region of low prevalence of HCV infection, estimates of data from the National Health Survey 2012 indicate that the prevalence of anti-HCV antibodies is 0.3% (95% CI, 0.13 to 0 67) in the population of 20-49 years.7 However, the prevalence of anti-HCV antibodies in the adult population of Mexican origin living in the USA is significantly higher than that reported for the population living in Mexico. For example, a study reported an anti-HCV antibodies prevalence of 2.3% (95% CI, 1.2-3.4) among economically disadvantaged Mexican Americans living in South Texas, USA.8 This differences observed between Mexican populations living inside and outside Mexico, may be related to environmental factors or differences in access to health care services between the populations.6
Epidemiological evidence shows that a wave of HCV infection occurred in Western countries particularly affected people who were born in the period 1945-1965.5 The Birth cohort 1945-1965 (so-called “baby boomers”) has been the goal of the Centers for Disease Control and Prevention of Diseases for mass screening because of the high prevalence of HCV in this age group, which has been estimated at 3.3%.9 In the USA, among the non-hospitalized civilian population, positive for HCV RNA, the prevalence of HCV infection was 1.0% (95% CI, 0.8 to 1.2). Infected persons are most likely to be aged 40-59 years, male, and non-Hispanic black, and lower educational level and family income.5
The National Health Survey in Mexico shows that prevalence of anti-HCV antibodies in adult Mexican population aged >20 years is 1.4% (95% CI, 1.1-1.6). However, the effect of age is evident from the generation born in the years 1953 and before, with a prevalence rate of 1.1% (95% CI, 0.7 to 1.5), 1.3 % (1.9 to 5.1), for groups of 40-49 and 50-59 years old; and 3.2% (1.6 to 4.8) and 2.1% (1.3 to 3.6) for groups of 60-69, and 70 years old, respectively.10
The international consensus recommends increasing the capacity for the detection and treatment of HCV as a cornerstone in many countries. This recommendation includes the assessment of cohort groups according to the year of birth as a useful tool for identifying and maximizing the use of resources in at-risk populations.11 Consistent with this perspective, the aim of this study was to document the differences in the prevalence of HCV infection in Mexico among different age groups, with a focus on birth-cohort or baby boomer population.
Material and MethodsStudy setting, population, and measurementsWe used a cross-sectional design, we included consecutive asymptomatic subjects > 18 years of age who were referred to our clinical pathology laboratory between July 2009 to June 2014 for serological evaluation of anti-HCV antibodies, referred by their doctors on suspicion of having HCV infection. We not present the results of the evaluation of the clinical condition of the patient or liver enzymes values. The serum samples from blood donors initially reactive to HCV and sent to the laboratory for the detection of HCV RNA were excluded.
Our hospital provides care to patients primarily middle- and high-income residents of Mexico City and the metropolitan area. The patients were stratified into three groups according to the year of birth: before 1945, 1945-1965, and 1966-1992.
Serum HCV antibody level was measured using a commercial chemiluminescence method (ARCHITECT, Abbott Laboratories. Abbott Park, Illinois, USA). HCV RNA level was measured in 152 patients using AmpliPrep/COBAS kits (Roche Molecular Diagnostics. Indianapolis, USA).
The study was approved by the Human Subjects Committee of the Medica Sur Clinic Foundation. Written informed consent was obtained from all participants before entry into the study.
Statistical analysisData were analyzed using IBM SPSS Version 17 statistical software. Standard descriptive statistics were obtained. The significance of comparisons between groups was assessed using a nonparametric test for K independent samples (Kruskal-Wallis) with a significance level of p < 0.05.
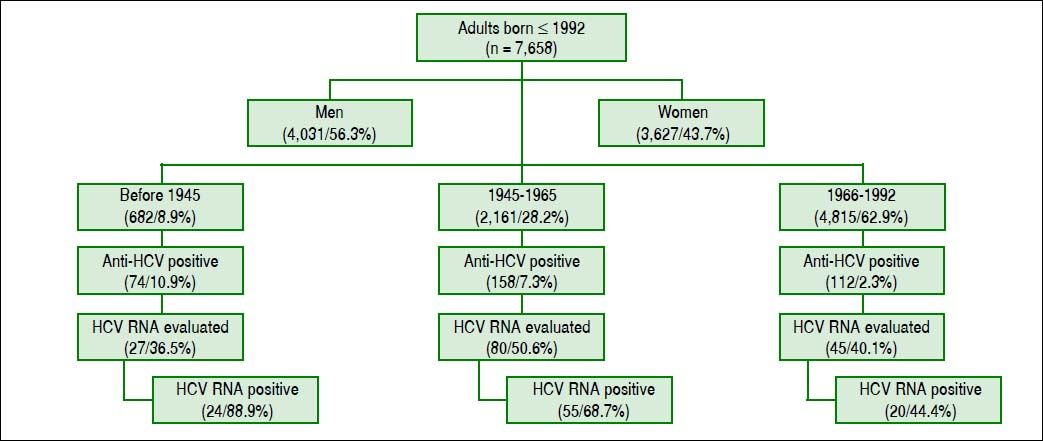
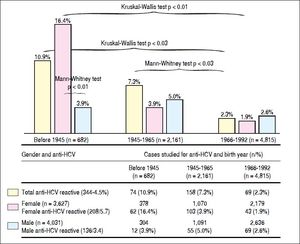
ResultsWe included 7,658 subjects, 4031 cases were male (56.3%) and 3627 women (43.7%). The numbers (percentages) of people according to the year of birth were 682 (8.9%) for those born before 1945; 2,161 (28.2%) for those born in 1945-1965, and 4,815 cases (62.9%) for those born in 1966-1992. The global prevalence of anti-HCV antibody reactivity was 4.5% (344/7,658) (Figure 1).
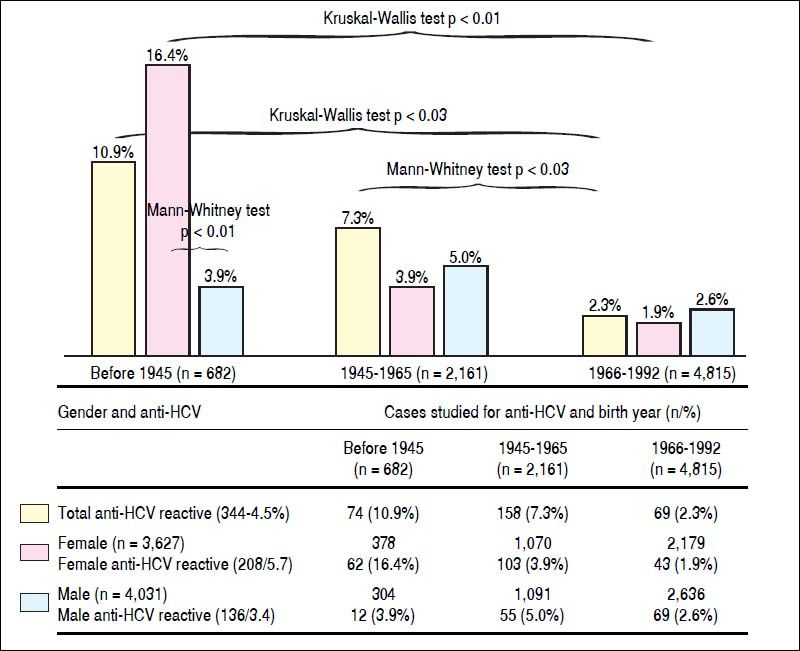
The frequencies of serological reactivity to HCV were 74 (10.9%), 158 (7.3%), and 112 cases (2.3%) (comparison between all groups, Kruskal-Wallis test p < 0.03), being more evident compared to those born between 1966-1992. The latter group had the lowest prevalence of anti-HCV reactivity (Figure 2).
Differences in reactivity to anti-HCV according to gender and year of birtfi. Tfie frequency of anti-HCV is statistically significant in the age group of patients born before 1945, being more evident compared to those born between 1966-1992 (10.0% vs. 7.3% and 2.3% Krusliai-Wallis p < 0.03). This statistical difference persists group compared 1945-1965 with the group of subjects born 1966-1992 (IMann-Whitney p < 0.03). The gender difference is aiso observed, women born before 1945, were four times more often ant-HCV than men in the same age group (Mann-Whitney p < 0.01). The frequency of ant-HCV in women decreases towards the younger age groups, with statisticaiiy significant differences (16.4%, vs. 3.9%, and 1.9%, Krusliai-Wallis p < 0.01) for remaining two age groups prevaience gender is reversed, being more frequent HCV in men than in women, but these differences are not statisticaiiy significant. HCV: hepatitis C virus.
The gender difference is also observed, women born before 1945, were four times more often anti-HCV than men in the same age group (16.4% vs. 3.9%. Mann-Whitney test p < 0.01). The frequency of anti-HCV in women decreases towards the younger age groups, with statistically significant differences (16.4% vs. 3.9% and 1.9%. Kruskal-Wallis test p < 0.01) for remaining two age groups prevalence gender is reversed, being more frequent HCV in men than in women, but these differences are not statistically significant, with 1.9% for women, and 2.6% for men (Figure 2).
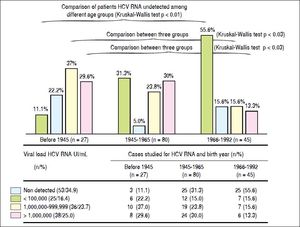
The HCV RNA level was quantified in 152 subjects, with 27 (36.5%), 80 (50.6%), and 45 (40.1%) patients born before 1945, 1945-1965, and 1966-1992, respectively (Figure 1). The respective numbers (percentages) of 99 people positive for HCV RNA (65.1%) were 27 (88.9%), 55 (68.8%), and 20 (44.4%) for each age group, and remaining cases were negative for HCV RNA detection.
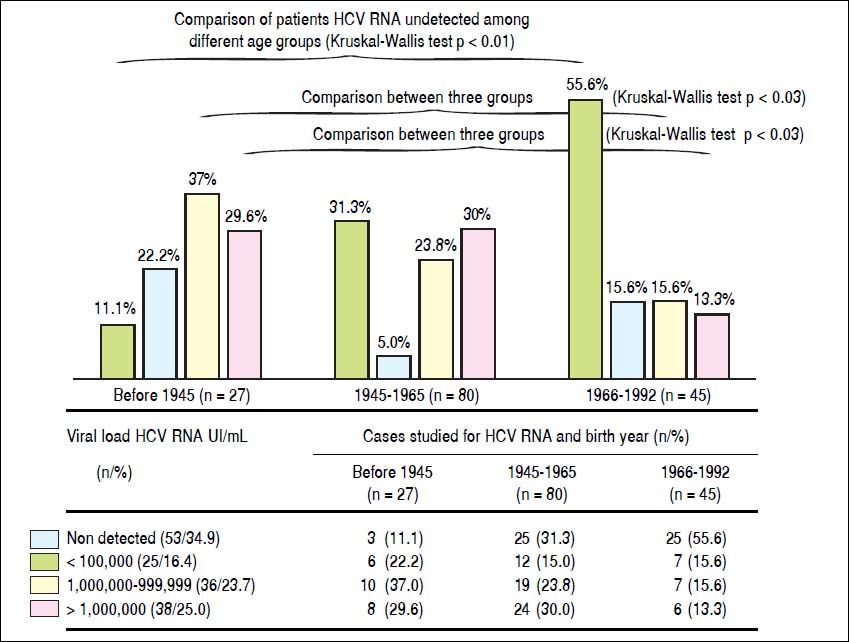
In patients with detectable HCV RNA, the viral load was < 100 000 IU/mL in 25 (16.4%), 100,000-999,999 IU/mL in 36 cases (23.7%), and > 1 million in 38 patients (25.0%). Similarly, patients born before 1945, they showed higher frequency of cases with high viral load (patients with more than 100,000 IU/mL HCV RNA), with statistically significant differences with the group born between the years 1966-1992 (Kruskal-Wallis p < 0.03) (Figure 3). Groups of older patients and anti-VHC, with year of birth before 1965, are more likely to show reactivity to HCV RNA and significant viral load (OR 10.0, CI 95% 4.8 to 20.1)
Viral load of HCV RNA. Comparison of patients HCV RNA undetected among different age groups, less frequently in the case group born before 1945 (11.1% vs. 31.3 and 55.6%, respectively Kruskal-Wallis p < 0.01). Similarly, patients born before 1945, they showed higher frequency of cases with high viral load (patients with more than 100,000 IU/mL HCV RNA), with statistically significant differences with the group born between the years 1966-1992 (Kruskal-Wallis p < 0.03). HCV:hepatitis C virus.
The trend in prevalence of anti-HCV shows a similar behavior to Mexico12 and Latin American countries,6 with minor occurrence in Bolivia and Panama (0.1%) and highest for Puerto Rico and Argentina (2.4%) and 0.8-1.6% for Mexico.13,14 Although our country is considered as a region of low prevalence of this infection, the impact on health system is highly significant because about a million people in Mexico have HCV markers and 16-80 % of them suffer of chronic hepatitis C.15
The prevalence of HCV infection and clinical stages of the disease are distributed unevenly in most countries. Clinicians have generally focused on certain risk groups or birth cohorts, but the risk may change over time, as the population is also growing and changing. People born before 1965, especially those born before 1945, have been identified as at-risk populations along with intravenous drug users, patients undergoing hemodialysis,16 and health workers,17,18 and mainly patients who received blood transfusion.19
It is also affected by the year anti-HCV antibodies universal screening was performed in blood donors.20 People most at risk are those who received a blood transfusion before 1992 (1993 in the case of Mexico), when health authorities established as a mandatory measure detecting HCV antibodies in all blood donations. In developing countries, the main risk factor is the blood transfusions, while in developed countries the use of intravenous drugs in the most important means of transmission.13 In blood donors were initially tested positive doubtful for antibodies to HCV,14 the most frequent risk factors identified were blood transfusion (36-42%) and < 10% of user drugs.21
Mexican blood donors older, they also have a different behavior in HCV infection. Documented an increased occurrence of serological marker, greater intensity in the reaction in the values of S/CO, most often in the identification of the HC-RNA and significant viral load HCV increase in blood donors more age.22 A similar behavior was observed in the results presented in our report for patients with clinical intent of the study, which supports the concept of the effects of HCV infection and birth cohort.
A “baby boomer or birth cohort effect” has been observed in different population groups in Latin America23 and Mexico. For example, in the state of Veracruz located on the Gulf of Mexico,24 the highest prevalence of anti-HCV antibodies was in blood donors born before 1975 (OR 1.43, 95% CI, 1.05-1.95). In the National Survey of Health and Nutrition 2012, an effect of age was not documented, but the prevalence rates of anti-HCV were 0.35% and 0.09% for those born before and after 1973, respectively.7 This may have been affected by bias in the selection of subjects younger than 50 years.
A gender gap, especially in subjects born after 1965, has been described inconsistently in the literature. In our report, there is a difference in the prevalence of HCV infection among men and women, the prevalence is higher in women born before 1945. In the African-American population,25 anti-HCV antibodies are found more often in men, but there is no difference between men and women when the presence of HCV is identified using molecular tests for HCV RNA.
However, we consider the possibility the effect on selection bias, since for blood donors, has been reported similar frequencies in the reactivity of anti-HCV with no gender difference.26
In our study, patients older than 60 years had a higher prevalence of HCV antibodies and were at high risk of chronic active hepatitis, as evidenced by the high rate of reactivity of HCV RNA (88.9%). These results support the concept that the age is an important predictor of HCV infection, and the risk increases with age in most clinical settings. This age group also has a poor response to antiviral treatment,17,27 greater likelihood of progression to liver disease, and more severe liver fibrosis as a specific pattern of clinical presentation.28 However, the most notable difference is the presence of HCV RNA in subjects located in the two older age groups. That is, the older subjects with HCV, are more likely to have reactivity to HCV RNA with significant viral load (OR 10.0, 95% CI 4.8 to 20.1). These data suggest that it may be necessary controlled clinical trials to implement specific preventive and therapeutic measures for patients with HCV in the elderly.17
In Mexico,22 from 34.2 to 66% of people with HCV antibodies were identified as positive HCV RNA.29 We observe similar figures in our report. However, there are important issues that have not been considered in our report, as is the diagnostic ability of commercial tests for the detection of anti-HCV and HCV RNA, as well as differences between analytical platforms for detection of HCV.30,31
The burden of disease caused by hepatitis is high in Latin America and the Caribbean.21 In 47% of countries in Latin America, offering the entire population testing hepatitis B virus and HCV, and 26% offered only to particular risk groups. Nevertheless, none of these countries includes the birth cohort as a risk group.31 Diagnostic tests of hepatitis B virus and hepatitis C, are mandatory for risk groups in 15% of countries.31,32 But without doubt, blood services are contributing the most significant number of new patients detected for HCV infection in Latin America.31
The available data suggest a wide variation in the prevalence of chronic HCV infection between countries in Europe. Countries in the south and east of the European Union have a much higher prevalence of chronic HCV infection (2-8%) compared with countries in northwestern Europe (< 1%).33 Among the 10 countries in Central and Eastern Europe that responded to a survey, the common features of many surveillance systems include mandatory surveillance, passive case finding, and reporting of both acute and chronic HCV infections. In many countries there are gaps in relation to screening policy for HCV, and no included birth cohort.34
In this report there are methodological limitations that must be taken into account for the correct interpretation and applicability of this information in the clinical setting. There is a selection bias in the inclusion of patients who do not represent the general population and over-represent subjects with higher expected frequency of IgG antibodies against HCV by the clinical intent of the study.
The study design does not allow the application of the results to the general population, and limits the assessment of the possible impact the association between age group and the higher prevalence of anti-HCV, which ultimately is the main constraint to justify need for detection of HCV in birth cohort function. Despite these limitations, the data presented in this study suggest the need for future research to expand on this topic.
ConclusionWe observed a high prevalence of chronic HCV infection unrecognized in this series of people born before 1945 and from 1945 to 1965; the prevalence was twice the population born from 1966 to 1992 period. In Mexico, there are no HCV screening programs in groups or high-risk population in general, including baby boomers. We need to establish approaches based detection risk should be extended to cohorts specific birth to improve the capture rate of those infected with HCV and on the basis of this information assess the impact on disease burden level national.
Abbreviations- •
CI: confidence interval.
- •
HCV RNA: hepatitis C virus RNA detected.
- •
HCV: hepatitis C virus.