Sorafenib has been the standard of care for first-line treatment of advanced hepatocellular carcinoma, a complex disease that affects an extremely heterogenous population. Thereby requiring multidisciplinary individualized treatment strategies that match the disease characteristics and the patients’ specific needs.
Material and methodsData for 175 patients who received sorafenib for hepatocellular carcinoma in three different hospitals in Sao Paulo, Brazil over a span of nine years were retrospectively analyzed.
ResultsThe median age was 62 years. Percentages of patients with Child-Pugh A, B and C liver cirrhosis were 61%, 31% and 5%, respectively. Approximately half of the patients had Barcelona Clinic Liver Cancer stage B disease, and the other half had stage C. The median treatment duration was 253 days. Sorafenib dose was reduced to 400 mg/day in 41% of the patients due to toxicity. Overall objective response rate as per Response Evaluation Criteria in Solid Tumors and its modified version was 39%. Patients who received transarterial chemoembolization (TACE) at any point during sorafenib therapy were significantly more likely to experience an objective response. After a median follow-up of 339 days, the median overall survival was 380 days. Child-Pugh cirrhosis, tumor response and concomitant chemoembolization were independent prognostic factors for overall survival in multivariate analysis.
ConclusionOur results suggest that, in experienced hands, sorafenib therapy may benefit carefully selected hepatocellular carcinoma patients for whom other therapies are initially contraindicated, including those patients with Child-Pugh B liver function and those patients who are subsequently treated with concomitant TACE.
Hepatocellular carcinoma (HCC) is the fifth most common tumor and approximately 700,000 people die for this disease each year worldwide, making it the third leading cause of cancer death.1 In some countries like the United State, HCC is the only cancer for which mortality is increasing2 due to the high prevalence of chronic hepatitis C and the epidemic of nonalcoholic fatty liver disease.
Since 2007, sorafenib has been the standard of care for the first-line treatment of advanced hepatocellular carcinoma (HCC) patients,1 based on statistically significant and clinically meaningful overall survival benefits observed in two phase III clinical trials,2,3 as well as a manageable toxicity profile, with diarrhea, hand-foot skin reactions, fatigue, rash/desquamation and anorexia being the most common drug-related adverse events.4
However, HCC is a particularly complex disease, often affecting an extremely heterogenous patient population, in terms of etiology of chronic liver disease, hepatic function, presence of comorbidities, and tumor burden.5 In addition, lack of access to different treatment modalities6,7 and poor communication between specialists8 may further accentuate this heterogeneity, thereby requiring multidisciplinary, individualized treatment strategies that match the disease characteristics and the patients’ specific needs.
The objective of this study was to evaluate the efficacy and safety of sorafenib in a truly broad population of HCC patients treated in the routine clinical practice of three separate hospitals in Sao Paulo, Brazil, and to draw conclusions about the use of sorafenib in different subgroups of patients.
Material and MethodsA retrospective chart review was performed for all patients treated with sorafenib for HCC by a single hepatologist in three different hospitals in Sao Paulo, Brazil, between December 1, 2007 and September 30, 2016. Medical records were used to gather patient demographic information, disease etiology, tumor staging, liver function, sites of metastasis, previous therapies, duration of treatment, and adverse events. Only patients with at least one follow-up visit or telephone call after treatment initiation were included. Informed consent was obtained from all subjects. The study was approved by the ethics committee on human research and followed the ethical principles of the Declaration of Helsinki.9
HCC diagnosis was based on histology, the criteria of the American Association for the Study of Liver Diseases1 and/or the criteria of the European Association for the Study of the Liver.10 The Barcelona Clinic Liver Cancer (BCLC) classification system11 was used for tumor staging, and the Child-Pugh score was used to assess liver cirrhosis. Sorafenib was administered orally at the standard initial dose of 400 mg twice daily, with a reduction in toxicity according to the standard protocol.2 Patients were clinically and radiographically evaluated at routine followup visits, which were typically every 3-4 weeks during sorafenib therapy. Both the Response Evaluation Criteria in Solid Tumors (RECIST)12 and the modified RECIST13 were used to assess tumor response. All data were collected as part of routine clinical practice.
Descriptive statistics were calculated for baseline demographic and clinical characteristics. Overall survival was calculated from the start of sorafenib treatment until death or last follow-up. Survival curves were estimated by the Kaplan-Meier method, and differences in survival were evaluated by the log-rank test with the use of the online application for survival analysis 2.14 We fitted the Cox proportional-hazards regression model to identify factors associated with survival, using the coxph function in the survival package for the statistical software R (R Core Team, 2016).
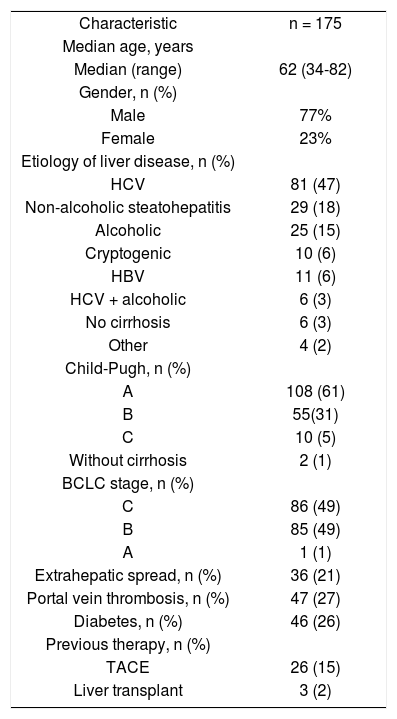
ResultsA total of 175 patients were included in this analysis. Table 1 shows baseline patient and disease characteristics. One hundred sixty-six patients (97%) had liver cirrhosis, and 3% in normal livers.The most common cause being hepatitis C virus, followed by alcoholism and hepatitis B virus. Other causes included coinfection with the human immunodeficiency virus (n = 3) and schistosomiasis (n = 1). Most patients were Child-Pugh A cirrhosis (61%) and BCLC stage B or C (49% each) HCC. The proportions of patients with portal vein thrombosis and extrahepatic metastasis were 27% and 21%, respectively. Approximately a quarter of the patients were diabetic. Previous therapies included transarterial chemoembolization (TACE; 15%) and liver transplant (2%).
Baseline patient and disease characteristics.
| Characteristic | n = 175 |
| Median age, years | |
| Median (range) | 62 (34-82) |
| Gender, n (%) | |
| Male | 77% |
| Female | 23% |
| Etiology of liver disease, n (%) | |
| HCV | 81 (47) |
| Non-alcoholic steatohepatitis | 29 (18) |
| Alcoholic | 25 (15) |
| Cryptogenic | 10 (6) |
| HBV | 11 (6) |
| HCV + alcoholic | 6 (3) |
| No cirrhosis | 6 (3) |
| Other | 4 (2) |
| Child-Pugh, n (%) | |
| A | 108 (61) |
| B | 55(31) |
| C | 10 (5) |
| Without cirrhosis | 2 (1) |
| BCLC stage, n (%) | |
| C | 86 (49) |
| B | 85 (49) |
| A | 1 (1) |
| Extrahepatic spread, n (%) | 36 (21) |
| Portal vein thrombosis, n (%) | 47 (27) |
| Diabetes, n (%) | 46 (26) |
| Previous therapy, n (%) | |
| TACE | 26 (15) |
| Liver transplant | 3 (2) |
The median treatment duration was 253 days (range, 111,188). The sorafenib dose was reduced to 400 mg/day in 72 patients (41%) due to toxicity. Among these patients, 12 had the dose subsequently increased to 600 mg/day and seven to 800 mg/day. Four patients did not tolerate the dose increase. All three patients previously subjected to liver transplant required dose reduction, and none had the dose increased. Diarrhea (31%), hand-foot skin reactions (27%), abdominal pain (14%), weight loss (13%), fatigue (13%), nausea (9%), vomiting (5%), hypertension (4%) and bleeding (4%) were the most common adverse events of any grade. One patient with Child-Pugh B cirrhosis and BCLC B tumor stage developed renal failure.
Eight patients achieved a complete tumor response (4.6%), and partial responses were observed in 61 patients (35%), for an overall objective response rate (ORR) of 39%. ORR did not differ significantly between ChildPugh A and Child-Pugh B/C patients (41% vs. 38%, respectively; p = 0.70), nor did it differ significantly with BCLC stage (A/B, 46.5% vs. C, 33.3%; p = 0.08). Twenty patients with objective response, three with disease stabilization and two with progressive disease underwent TACE at some point during sorafenib therapy. Patients who underwent concomitant TACE had significantly higher ORR than patients who did not (P < 0.001).
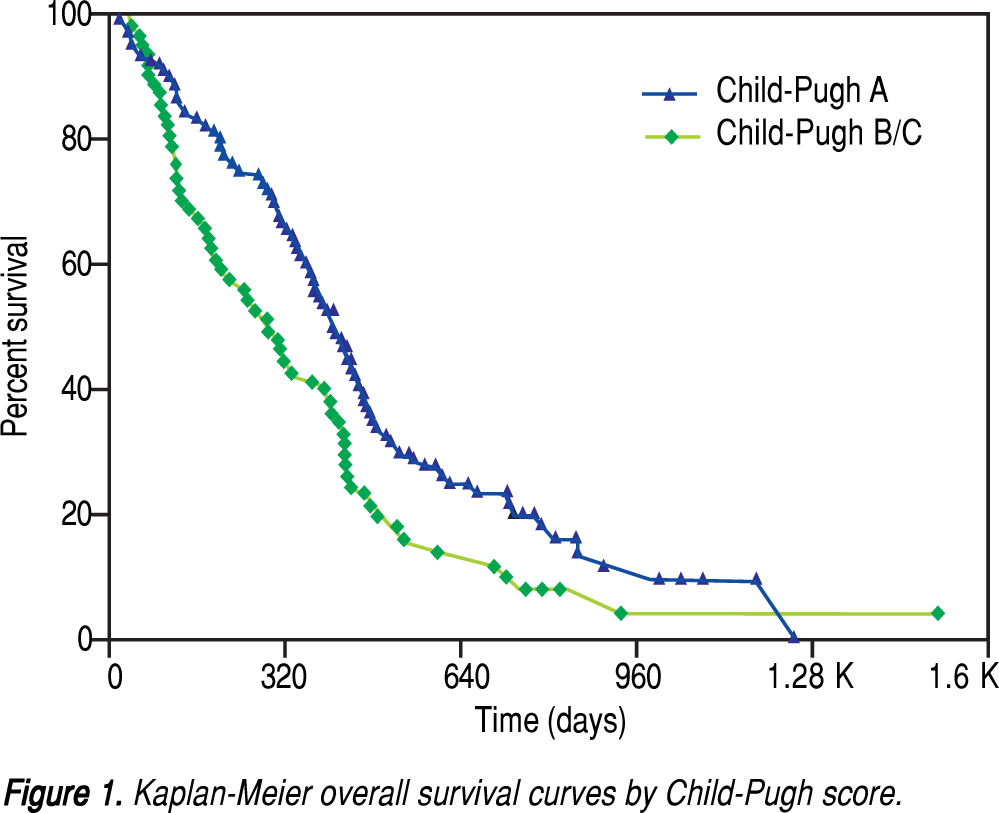
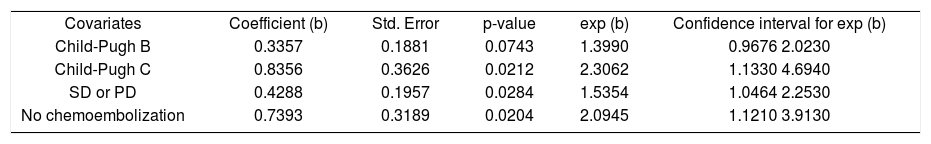
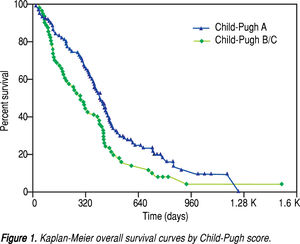
After a median follow-up of 339 days (range, 11-1524), the median overall survival (OS) was 380 days (95% confidence interval [CI], 318-424 days). The oneand two-year OS rates were 52.4% and 16.9%, respectively. As shown in figure 1, the median OS was significantly longer in ChildPugh A patients (410 days; 95% CI, 362-455 days) than in Child-Pugh B/C patients (285 days; 95% CI, 188-396 days; P = 0.0157). Multivariate stepwise Cox regression revealed that Child-Pugh cirrhosis, tumour response and concomitant TACE were independent prognostic factors for overall survival (Table 2).
Regression coefficients, standard errors, p-values, exponentiated coefficients and 95% confidence intervals for the association between survival times and the covariates Child-Pugh, response and transarterial chemoembolization.
| Covariates | Coefficient (b) | Std. Error | p-value | exp (b) | Confidence interval for exp (b) |
| Child-Pugh B | 0.3357 | 0.1881 | 0.0743 | 1.3990 | 0.9676 2.0230 |
| Child-Pugh C | 0.8356 | 0.3626 | 0.0212 | 2.3062 | 1.1330 4.6940 |
| SD or PD | 0.4288 | 0.1957 | 0.0284 | 1.5354 | 1.0464 2.2530 |
| No chemoembolization | 0.7393 | 0.3189 | 0.0204 | 2.0945 | 1.1210 3.9130 |
Our patient population differs from those of most prospective and retrospective studies in several aspects. A greater proportion of patients had intermediate-stage HCC, which represents an optimization of the ‘treatmentstage migration approach’,10 a concept well known in the field of Oncology.
Before the publication of the new E.A.S.L. guideline, the BCLC did not discuss about an alternative treatment for those patients who did not respond to initial treatment, a common concept among oncologists. For this reason, we treated patients with intermediate stages who did not respond to TACE with Sorafenib.
By the time the patients from the data gathered for this paper were treated, we started Sorafenib based upon clinical judgment, even if the patient had a Child-Pugh C liver function. Nowadays, we start this medication only to Child B patients with good performance status and we do not treat Child C patients anymore. Based on our learning curve among the years, this seems to be the best strategy for patients with advanced liver function. We know that in real life our patients are different from those of the ones in randomized clinical trials.
The percentage of extrahepatic spread was lower, indicating the use of sorafenib in a population with less advanced disease.
This is a retrospective analysis, so we have some missing data about the side effects and nowadays we know that they are good predictors of response, as Maria Reig showed on her paper in the Journal of Hepatology.15 In our data, we found that 60% of our patients experienced some degree of side effects, out of these patients, 15% had to stop treatment for side effects.
The median OS observed in our cohort -380 dayscompares favorably with the results obtained in other retrospective studies, in which OS times ranged from 141 to 336 days.16-23 Although this finding could be attributed to a more customized treatment approach, it could also be due to selection bias. Restricting the inclusion criteria to patients with at least one follow-up or phone call after treatment initiation may have excluded patients with bad prognosis and/or early death, thereby skewing the results towards a more favorable population. In addition, our study included 25 patients treated with concomitant TACE. In other series, patients who received concomitant TACE were shown to have less advanced disease and longer OS than patients who did not receive concomitant therapy24
In addition, performing TACE during sorafenib therapy may have positively impacted ORR as well. We did in a few patient combined treatment, TACE and Sorafenib, before the publication of the results os SPACE trial wich, the ORR in the sorafenib plus TACE arm was 55.9%,25 whereas in the SHARP and Asia-Pacific trials, the ORRs in the sorafenib arm were 2.0% and 3.3%, respectively.2,3 Our study showed an ORR of 39%, and 20/61 patients with an objective response received concomitant TACE.
The use of both mRECIST and RECIST to evaluate tumor response may have also played a role in our results, since the combination may better assess response during HCC treatment. Nevertheless, the high ORR observed in our study may have resulted from imaging reader bias, as the scans were evaluated by the investigator himself and were not blindly reviewed.
Despite all shortcomings, our results suggest that sorafenib may indeed benefit some patients for whom other therapies are contraindicated. The earlier use of sorafenib in the disease course must, therefore, be considered, and with adequate surveillance, even patients with Child-Pugh B liver function can gain from treatment. The appropriate management of adverse events is paramount to minimize sorafenib dose reduction or discontinuation. Taken together, these observations appear to support a multidisciplinary, individualized approach to HCC patients who are potential candidates for sorafenib therapy.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer.
- •
CI: confidence interval.
- •
HCC: Hepatocellular carcinoma.
- •
ORR:objective response rate.
- •
OS:overall survival.
- •
RECIST: response evaluation criteria in solid tumors.
- •
TACE: transarterial chemoembolization.
The authors declares that there is no conflict of interest regarding the publication of this article.