Background and aim: Near 25% of people infected with Human Immunodeficiency Virus (HIV) are also carriers of the Hepatitis C Virus (HCV). Hepatitis C virus infections are generally asymptomatic, and because HIV-infected individuals have a decreased immune response, these infections can escape immune control and lead to chronic asymptomatic disease. The use of direct-acting antivirals (DAAs) decreases the inflammation and liver fibrosis in this group of patients. Aim: To describe the characteristics of patients coinfected with HIV with HCV and analyze the changes in liver inflammation assessed by aminotransferases and liver fibrosis measured by transitional elastography.
Material and methods: Cross-sectional, retrolective, analytical and comparative study. We included elderly subjects with a diagnosis of chronic HCV infection coinfected with HIV, treated with DAAs and who had a viral load result 12 weeks after treatment, aminotransferases and basal transition and post-treatment elastography. Descriptive statistics and group comparison were performed with the Student's t test, and the Wilcoxon test was shown to show differences.
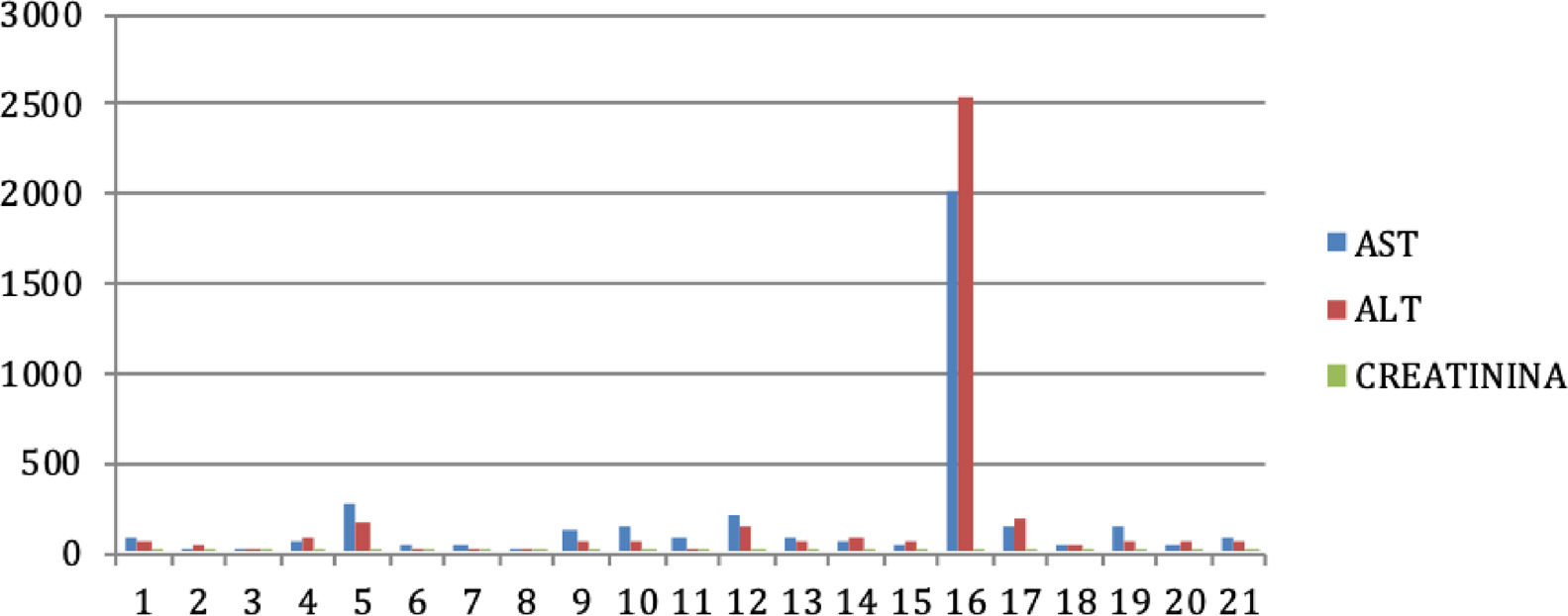
Results: 21 male subjects were analyzed, the mean age was 44±12.3 and 66% (14) were genotype 1 and 34% (7) genotype 4. The median AST 77 (42-1459) and ALT was 64 (35-87). The median fibrosis by transitional elastography was 6.5 (4.1-12.3). 100% percent of the participants received sofosbuvir and ledipasvir. The SVR was 95% in the analyzed group. The decrease in fibrosis measured by elastography before and after treatment was not statistically significant. There is a decrease in aminotransferases after treatment with AAD (Table 1).
Conclusions: Treatment with ADD in patients coinfected with HIV and HCV has SVR rates similar to those described in monoinfected patients (95% in our group) and decreases inflammation and fibrosis as measured by transitional elastography.
Conflicts of interest: The authors have no conflicts of interest to declare.