Healthy controls are subjects without the disease being studied but may have other conditions indirectly affecting outcome. In the present epidemics of obesity a few subjects with undiagnosed nonalcoholic fatty liver disease enter clinical studies as controls, producing biased results. Stricter selection criteria should be considered to prevent this risk.
How healthy are “healthy volunteers”? An important but difficult question that raises a number of related intriguing issues for evidence-based medicine (EBM). By definition, in a clinical study a “healthy” control is a person who does not have the disorder or the disease being studied, but may have other disorders that are not addressed in the specific setting of the research. As an example, subjects treated for hypertension might be confidently used as “healthy” controls in studies measuring bowel habits or skeletal muscle fitness in comparison to subjects with inflammatory bowel diseases or following orthopedic surgery, respectively. But they are not “healthy” and could also have diseases possibly affecting the outcome of the clinical study. Let's consider a patient with rheumatoid arthritis –a disease known to increase cardiovascular risk– included as control in a study assessing cardiovascular outcomes. The results might be biased.
If we limit the “healthy” state to subjects who are free from any possible disease, we fall into other difficulties. Individuals who do not have any demonstrable disease or disorder belong to a category of so-called “supernormal” subjects. Using these individuals in comparison to diseased patients frequently leads to overestimate differences, or to measure differences that are not clinically relevant. Finally, using a population-based approach as control group provides measures of the health state in the community, inclusive of subjects who deviate from the norm. Consequently, differences might be reduced to a minimum or become insignificant. Any compromise has advantages and disadvantages that should be accurately balanced during study planning.
Also the term “volunteers” creates difficulties. We read in the Oxford Learning dictionary that a volunteer is “a person who freely offers to take part in an enterprise or undertake a task” or “a person who works for an organization without being paid”. This is not the case of “volunteers” in clinical studies, who are paid in several Countries –including the U.S. where most studies funded by the National Institute of Health are run–, or accept to volunteer to obtain benefits (free tests, drugs or treatment). This issue can potentially lead to over-recruitment of subjects willing to undertake clinical controls because at risk for diseases (e.g., familiarity for chronic-degenerative diseases) or with diseases unrelated to the trial. The definition of their “healthy” state is left to their reporting of present and/or previous diseases vs. health state and/or to a battery of tests that should fall into “normal ranges”.
But what are “normal” ranges? A critical example comes from the definition of “normal” alanine aminotransferase levels (ALT), a pivotal test to define the metabolic processes occurring in the liver as “normal”. The upper normal limits of ALT have long been set to 40 U/L, based on the upper 95% confidence interval of values measured in studies conducted in the ‘80s on subjects (usually blood donors) without overt liver disease or hepatitis B virus in-fection.1 These populations, however, were later shown to include subjects with hepatitis C as well as subjects with non-alcoholic fatty liver disease (NAFLD), artificially raising the safe upper limits of ALT. When patients with HCV infection were excluded, the “normal” ranges were reduced to 30 U/L in males and as low as 19 in females.2 What about NAFLD cases?
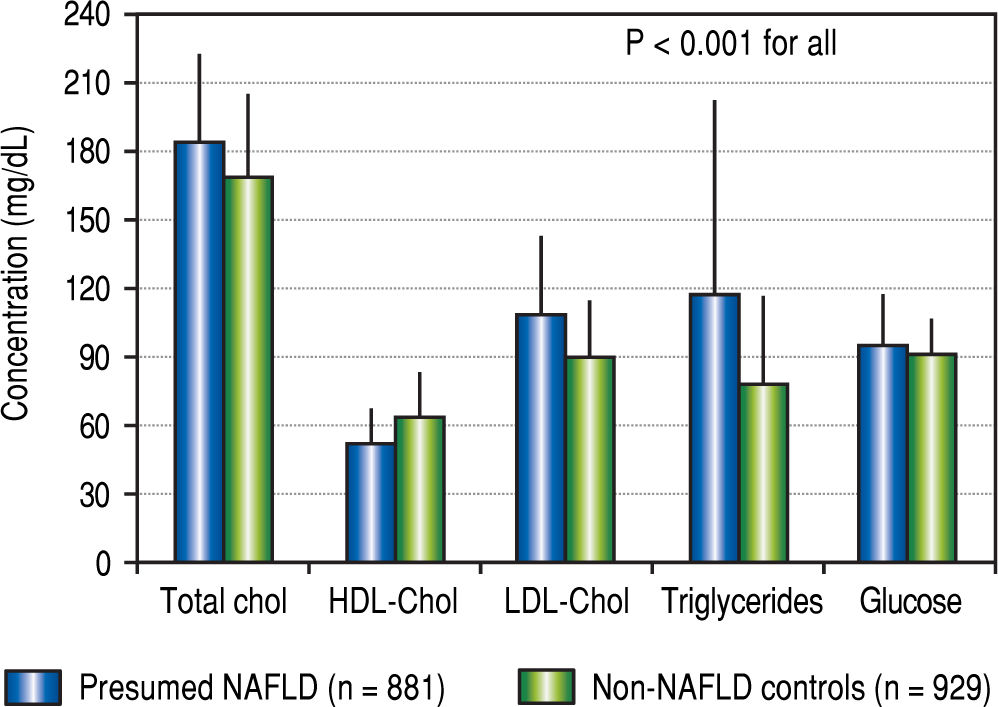
In a recent issue of Hepatology, Takyar, et al. tested the penetrance of NAFLD cases as “healthy volunteers” in the biomedical literature between 2011 and 2015.3 NAFLD was presumed in the presence of ALT above the updated ranges and body mass index (BMI) in the overweight/ obesity range; healthy non-NAFLD controls were subjects with normal ALT and BMI, whereas subjects with either ALT or BMI above the “healthy” definition were considered indeterminate. The validity of the studies according to the presence of presumed NAFLD (pNF) cases was estimated on the basis of the research question. Of 3,160 subjects participating as healthy volunteers in 149 clinical trials, 881 were classified as pNF (27.9%), leaving 929 (29.4%) as healthy non-NAFLD controls, and 1,350 (42.7%) as indeterminate. On average, pNF were older than healthy non-NAFLD controls, and their lipid and glucose profiles were characterized by abnormalities associated with the metabolic syndrome (Figure 1). This was expected to have a likely impact on study validity in 10 studies and a probable impact in other 41, i.e. on a total of 34% of tested trials. In the course of the trials, pNF subjects were more likely to show persistent ALT elevations, which were clinically significant in a few instances and could impact on safety analyses. Of note, the NAFLD fi-brosis score was in the range of advanced fibrosis in 6/694 pNF cases.
Average concentrations of lipid and glucose in subjects with presumed NAFLD and in non-NAFLD controls enrolled into studies as “healthy” volunteers. Data are presented as means and standard deviation. Redrawn from data published in Takyar, et al.3
The study outlines the difficulties in having reliable “healthy” controls for research purposes. Consider that the Takyar research possibly underestimates the impact of NAFLD. A significant proportion of subjects classified as indeterminate might indeed have NAFLD, based on their elevated BMI in the presence of normal ALT. A long series of reports have convincingly demonstrated that both isolated overweight/obesity (so called “metabolically healthy obesity” – but are these individuals really “healthy”?) or an isolated increase in ALT do not necessarily exclude NAFLD,4 and even severe, progressive NASH.5 The so-called “lean” NAFLD accounts for 10-15% of all NAFLD cases in most clinical series. Liver impairment in these subjects is probably driven by largely unidentified gene polymorphisms, producing an impact in metabolic functions that is definitely unpredictable.
The history of “healthy” controls in EBM is interesting and dates back to the early 20th century, when “healthy” individuals from prisons and military camps – which could thus obtain benefits from government–, or medical students and people with some family relationship with the researchers were used in clinical studies. Later, healthy individuals with no prior relationship with the researchers were recruited from outside State institutions through advertising and compensated for their participation into the study. Before entering a clinical study, they usually undergo a clinical and biochemical evaluation, but the possibility of subclinical metabolic impairment associated with overweight/obesity is rarely ruled out. Recruitment of these subjects in trials jeopardizes the validity of studies involving insulin resistance, li-pid metabolism, energy metabolism and associated outcomes in multiple ways. Overweight-associated, undetected NAFLD is expected to modify the metabolism and bioavailability of drugs,6 and to increase the prevalence of complications7 –thus reducing the rate difference in comparison to disease states– and has the potential to increase possible side-effects.
In conclusion, unless validated and cheap tests become easily available for a positive diagnosis of NAFLD, stricter criteria should be used to select “healthy” controls for clinical trials involving areas of research where the presence of NAFLD cases could impair the final results. Evidence-based medicine needs solid criteria to provide solid and reliable data; considering the epidemics of obesity, the shortage of potential candidates as “controls” is likely to create more and more difficulties in the future.
Funding SourcesThe work did not receive any specific funding. FM is supported by a research contract from DIMEC, University of Bologna, Italy.