Limited data describe current SBP epidemiology and specific secondary SBP prophylactic regimens, leading to variable prescribing practices. This work aims to compare 90-day and one-year SBP recurrence and mortality based on secondary SBP antibiotic prophylaxis regimens.
Materials and methodsWe performed a retrospective cohort of patients >18 years with an SBP diagnosis from 2010 to 2015 at two academic institutions. Eligible patients had ascitic PMN counts ≥250cells/mm3 or a positive ascitic culture. Patients were compared based on secondary SBP prophylaxis regimens (i.e., daily, intermittent, or no prophylaxis).
ResultsOf 791 patients with ascitic fluid samples, 86 patients were included. Antibiotic prophylaxis included daily (n=34), intermittent (n=36), or no prophylaxis (n=16). Nearly half of SBP episodes had a positive ascitic fluid culture; 50% were gram-negative pathogens, and 50% were gram-positive pathogens. Daily and intermittent regimens had similar rates of recurrence at 90-days (19.4% vs. 14.7%, p=0.60) and one-year (33.3% vs. 26.5%, p=0.53). Similarly, mortality did not differ among daily and intermittent regimens at 90-days (32.4% vs. 30.6%, p=0.87) or one-year (67.6% vs. 63.9%, p=0.74). When comparing any prophylaxis vs. no prophylaxis, there were no differences in 90-day or one-year recurrence or mortality.
ConclusionsIn patients with a history of SBP, our data indicate similar outcomes with daily, intermittent, or no secondary antibiotic prophylaxis. With available data, including ours, demonstrating a changing epidemiology for SBP pathogens, further data is required to determine if traditional approaches to secondary SBP prophylaxis remain appropriate.
Spontaneous bacterial peritonitis (SBP), an infection of the ascitic fluid without evidence of an intra-abdominal source, is the most common infection in patients with cirrhosis [1,2]. In patients who survive an episode of SBP, the risks of recurrence and mortality at one year are high at 69% and 62%, respectively [3].
Several studies assessing SBP prophylaxis, including secondary prophylaxis and mixed populations including primary and secondary prophylaxis, have demonstrated that chronic antibiotic use decreases SBP recurrence and extraperitoneal infections, establishing the benefit of antibiotic prophylaxis in patients at high risk for development of SBP [4–7]. Initial studies evaluating once weekly ciprofloxacin and five-times weekly trimethoprim/sulfamethoxazole (TMP/SMX) observed decreased SBP recurrence, suggesting that intermittent dosing strategies may be cost-effective alternatives to daily antibiotic prophylaxis regimens [6,7]. However, additional data reported incomplete intestinal decontamination with intermittent administration, and these researchers proposed that weekly dosing of ciprofloxacin should not be used due to the potential for increased antibiotic resistance [8].
Traditionally, the most common pathogens associated with SBP include Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae[9]; however, recent reports have revealed microbiologic changes in SBP-causing pathogens with increasing rates of gram-positive bacteria, fluoroquinolone (FQ)-resistant bacteria and multidrug resistant (MDR) bacteria leading to empiric antibiotic treatment failure [10–17].
Current guidelines recommend chronic antibiotic prophylaxis with daily norfloxacin, a poorly-absorbed FQ antibiotic with selective activity for gram-negative bacteria, for secondary SBP prophylaxis to decrease SBP recurrence and mortality [9]. Norfloxacin is not available in the United States; therefore, antibiotics with similar spectrums of activity (i.e., ciprofloxacin, TMP/SMX) are recommended alternatives.
Guidelines have incorporated concerns related to antibiotic resistance by preferring daily over intermittent antibiotic regimens [9], although data remain limited to support specific antibiotic regimens available to clinicians in the United States. Furthermore, in contrast to norfloxacin, both ciprofloxacin and TMP/SMX have high bioavailability after oral administration, further prompting concern over chronic antibiotic exposure and development of bacterial resistance.
Studies assessing secondary prophylaxis are restricted by small sample size, limited follow-up, or intervention with antibiotics no longer available in the United States [4,6,8,18]. As studies supporting secondary SBP prophylaxis approach 20 years, it is important that we assess current regimens used today to evaluate epidemiologic patterns and long-term outcomes of secondary SBP prophylaxis regimens. Therefore, we retrospectively evaluated long-term outcomes of daily, intermittent, or no prophylaxis in patients with a history of SBP for recurrence and mortality at 90 days and one year. Based on previous literature as well as guideline-supported recommendations, we hypothesized that patients receiving daily SBP prophylaxis would have improved outcomes, such as decreased recurrence and mortality, compared to intermittent SBP prophylaxis.
2Materials and methodsWe performed a retrospective cohort study at two academic medical centers in San Antonio, Texas (the University Health System University Hospital and the South Texas Veterans Health Care System Audie L. Murphy Memorial Hospital) comparing SBP prophylaxis regimens in patients with a history of SBP for both SBP recurrence and mortality at 90 days and one year. Initial SBP prophylaxis antibiotic regimens were used to categorize patients into three groups as follows: daily dosing, intermittent dosing (e.g., ciprofloxacin once weekly or TMP/SMX five times weekly), and no prophylaxis. The prophylactic antibiotic regimen used to categorize patients was the initial regimen initiated at hospital discharge or outpatient clinic visit following the first SBP episode, regardless of subsequent changes. Data were collected from electronic medical records using Microsoft® Office Access. Demographics, past medical history, medication use, Model for End-Stage Liver Disease Sodium (MELD-Na) score, laboratory values, microbiology data, hospital length of stay, transplantation, SBP recurrence and mortality data were collected. The study was approved by the research departments of University Health System and South Texas Veterans Health Care System and the institutional review boards of the University of the Incarnate Word and UT Health San Antonio. A waiver of informed consent was obtained.
2.1DefinitionsBaseline laboratory values were defined as the first recorded values from the day of SBP diagnosis (i.e., the day of paracentesis) or the closest available laboratory values from the time of diagnosis. Similarly, baseline outpatient medications were those present on the day of SBP diagnosis. History of varices or gastrointestinal bleeding were defined as a documented complication in the medical record or by the endoscopy imaging record as available. Hepatic encephalopathy was identified by a documented complication in the medical record or by the presence of lactulose in the medication reconciliation record. Serial paracenteses were defined by at least one procedure every two weeks [9]. SBP episodes were considered community-acquired if the paracentesis was performed within 48h of admission; alternatively, SBP episodes were considered hospital-acquired if the paracentesis was performed greater than 48h after hospital admission. SBP episodes were further classified into three categories as follows: SBP was defined by an ascitic fluid polymorphonuclear leukocyte (PMN) count ≥250cells/mm3 and positive ascitic fluid culture; culture-negative neutrocytic ascites (CNNA) was defined by PMN count ≥250cells/mm3 and negative ascitic fluid culture; bacterascites was defined by PMN count <250cells/mm3 and positive ascitic fluid culture.
2.2Inclusion and exclusion criteriaPatients were identified by review of ascitic fluid analyses performed between January 2010 and November 2015. Patients 18 years or older with an SBP diagnosis were eligible for inclusion. Patients were excluded if they had a suspected abdominal source of infection (i.e., secondary peritonitis), non-cirrhotic ascites (e.g., malignant ascites), or if placed on comfort care or expired within seven days of SBP diagnosis. Finally, patients were excluded if they were pregnant or incarcerated. In patients meeting inclusion criteria, data was collected from first SBP episode until death, loss to follow-up, or at least one-year from inclusion date as appropriate.
2.3OutcomesThe primary outcomes were SBP recurrence and mortality at 90-days and one-year in patients who received daily vs. intermittent antibiotic prophylaxis. Other outcomes included SBP recurrence and mortality at 90-days and one-year in patients with no prophylaxis as well as recurrence and mortality in patients receiving any antibiotic prophylaxis, daily or intermittent, vs. no prophylaxis. Microbiology data was used to identify SBP pathogens, concurrent bacteremia and pathogen susceptibility to antibiotic prophylaxis regimens.
2.4Data analysisPatient demographics, clinical characteristics, and outcomes were summarized with descriptive statistics. The Shapiro–Wilk W test was used to assess continuous variables for normality. Continuous data with a normal distribution was reported as mean and standard deviation and analyzed with the Student's t-test; continuous data with a non-normal distribution was reported as median and interquartile range and analyzed with the Wilcoxon rank-sum test. Nominal variables were analyzed using chi-square or Fisher's Exact, as appropriate. Survival curves were constructed for time-to-event variables (time to SBP recurrence, time to death) with the Kaplan–Meier method. Multivariable logistic regression and Cox proportional hazards analysis were performed to determine variables independently associated with risk of SBP recurrence and death at one-year. A p-value less than 0.05 was considered statistically significant. A sample size calculation was not performed; a convenience sample of all patients meeting inclusion criteria at the institutions was included in the cohort. Statistical analyses were performed using JMP 11.2.0® (SAS Institute Inc., Cary, NC, USA).
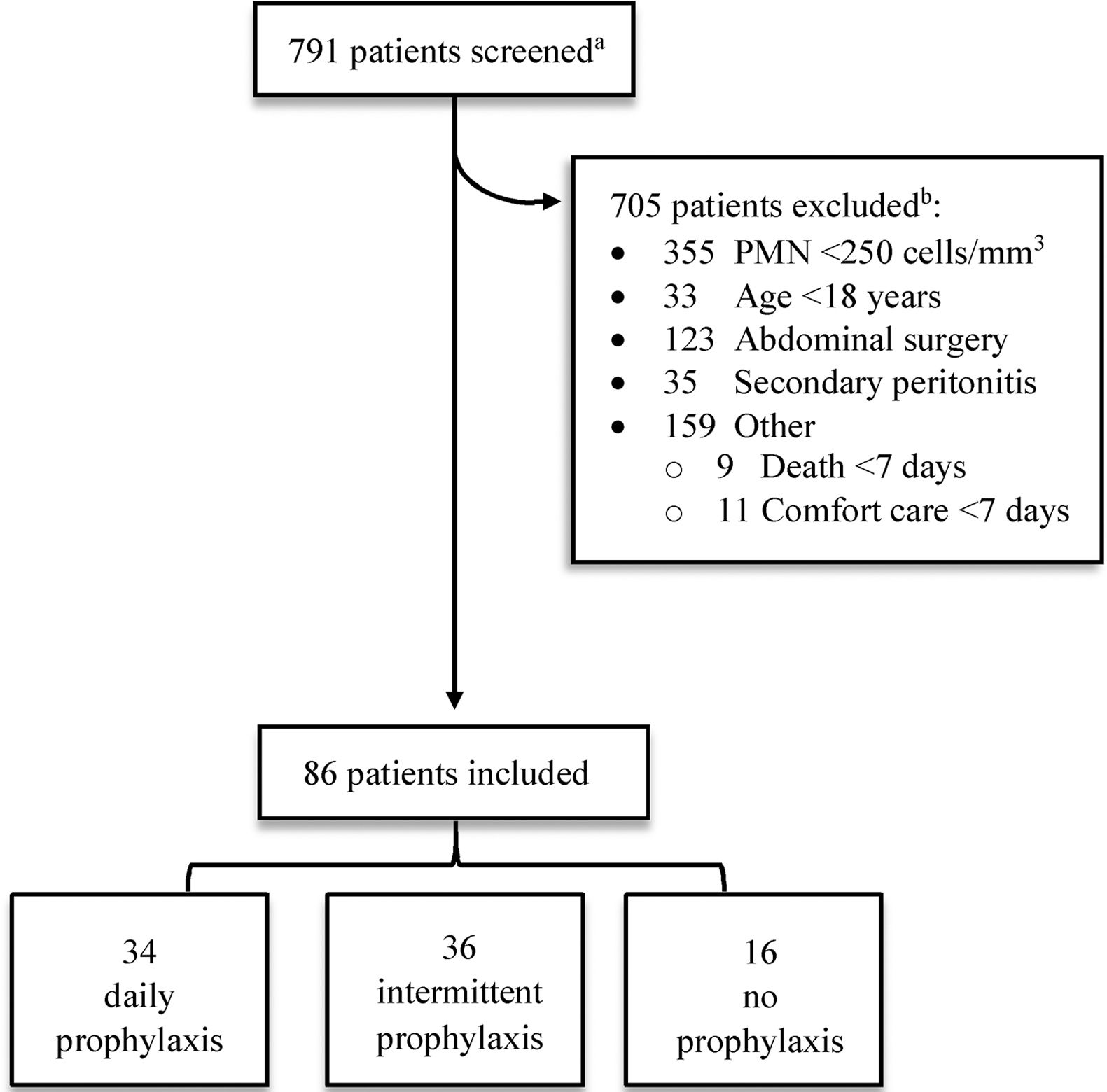
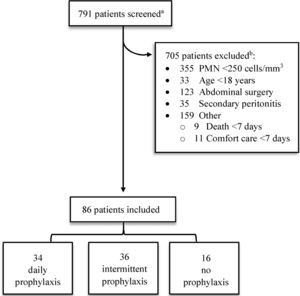
3Results3.1Patient characteristicsOf the 791 patient charts reviewed, 705 were excluded (Fig. 1). Half of these patients (50.4%) were excluded due to PMN count <250cells/mm3 without a positive ascitic fluid culture, 123 patient samples (17.4%) were collected during abdominal surgeries (e.g. bowel resection, appendectomy) and not obtained from ascitic fluid, and 35 patients (5.0%) had evidence of an abdominal source of infection (i.e. secondary peritonitis). Other causes for exclusion included death or comfort care within seven days of SBP diagnosis (2.8%), non-cirrhotic ascites (10.5%), and laboratory malfunction (6.0%). One patient with a history of liver transplant in 1989 was excluded due to chronic TMP/SMX therapy for Pneumocystis jirovecii pneumonia prophylaxis. A complete list of exclusions may be found in Appendix A.
Screening and eligibility.
PMN, polymorphonuclear leukocyte.
aPatients were identified by review of ascitic fluid analyses performed between January 2010 and November 2015.
bComplete list of exclusion criteria can be found in Appendix A.
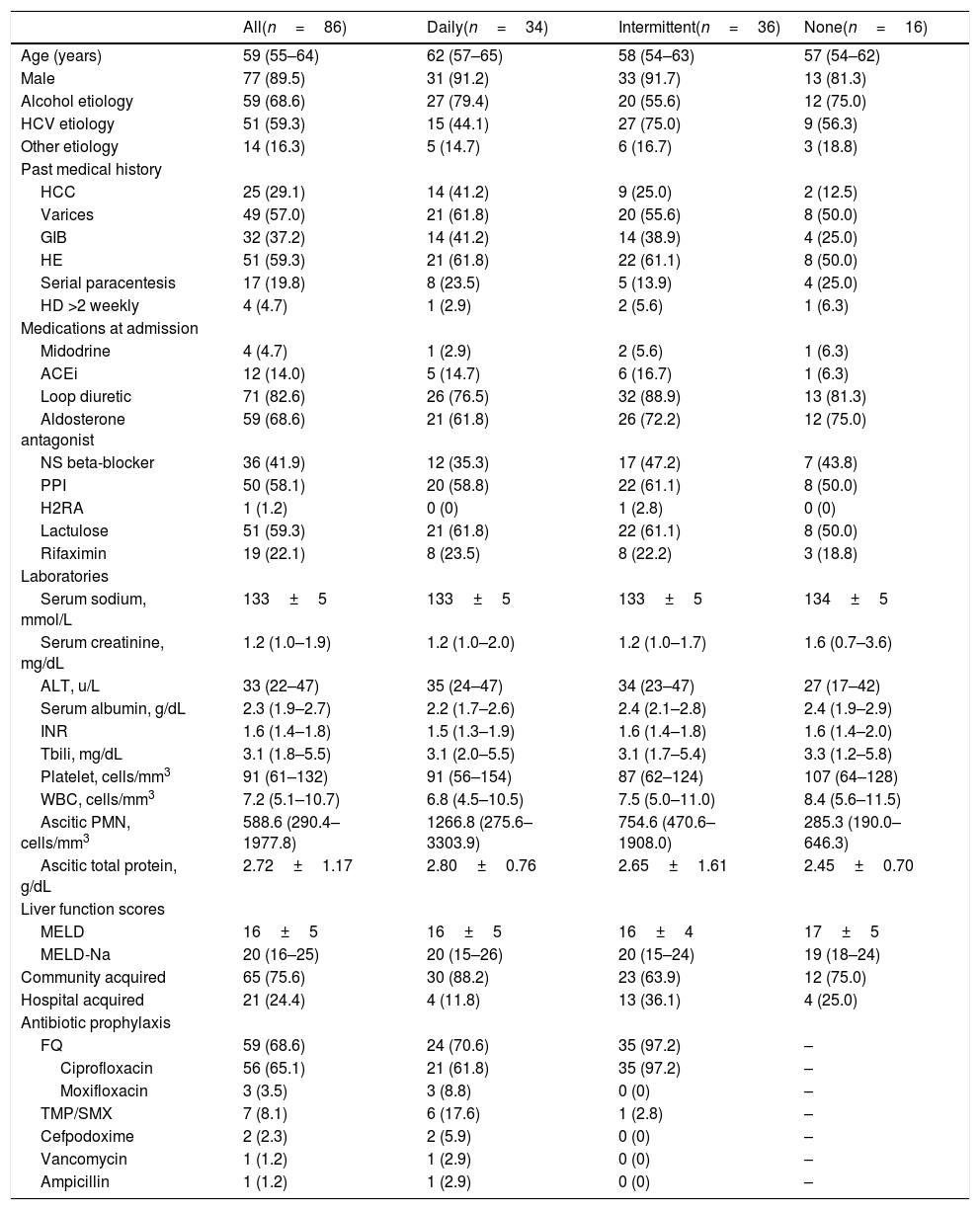
Eighty-six patients were included in the final cohort. Comparisons of patient characteristics are presented in Table 1. Daily and intermittent antibiotic prophylaxis regimens were most prevalent with 34 and 36 patients, respectively. Sixteen patients did not receive antibiotic prophylaxis. Most (65.1%) patients received antibiotic prophylaxis with ciprofloxacin. Patients were more likely to receive a FQ with intermittent dosing compared to daily dosing (97.2% vs. 70.6%, respectively). Daily dosing had more variable antibiotic regimens. Specifically, other daily antibiotic regimens included ciprofloxacin 250–500mg (61.8%), TMP/SMX 160mg/800mg (17.6%), moxifloxacin 400mg (8.8%), and cefpodoxime 100–200mg (5.9%). One patient received intravenous (IV) vancomycin post-hemodialysis three-times weekly for Enterococcus faecium cultured from ascitic fluid until transitioning to comfort care. Another received daily IV ampicillin for Enterococcus faecalis cultured from ascitic fluid and blood with related endocarditis until death. One patient received ciprofloxacin daily for primary SBP prophylaxis due to low ascitic fluid protein <1.5mg/dL; all other patients were receiving chronic antibiotics for secondary SBP prophylaxis.
Baseline characteristics.
| All(n=86) | Daily(n=34) | Intermittent(n=36) | None(n=16) | |
|---|---|---|---|---|
| Age (years) | 59 (55–64) | 62 (57–65) | 58 (54–63) | 57 (54–62) |
| Male | 77 (89.5) | 31 (91.2) | 33 (91.7) | 13 (81.3) |
| Alcohol etiology | 59 (68.6) | 27 (79.4) | 20 (55.6) | 12 (75.0) |
| HCV etiology | 51 (59.3) | 15 (44.1) | 27 (75.0) | 9 (56.3) |
| Other etiology | 14 (16.3) | 5 (14.7) | 6 (16.7) | 3 (18.8) |
| Past medical history | ||||
| HCC | 25 (29.1) | 14 (41.2) | 9 (25.0) | 2 (12.5) |
| Varices | 49 (57.0) | 21 (61.8) | 20 (55.6) | 8 (50.0) |
| GIB | 32 (37.2) | 14 (41.2) | 14 (38.9) | 4 (25.0) |
| HE | 51 (59.3) | 21 (61.8) | 22 (61.1) | 8 (50.0) |
| Serial paracentesis | 17 (19.8) | 8 (23.5) | 5 (13.9) | 4 (25.0) |
| HD >2 weekly | 4 (4.7) | 1 (2.9) | 2 (5.6) | 1 (6.3) |
| Medications at admission | ||||
| Midodrine | 4 (4.7) | 1 (2.9) | 2 (5.6) | 1 (6.3) |
| ACEi | 12 (14.0) | 5 (14.7) | 6 (16.7) | 1 (6.3) |
| Loop diuretic | 71 (82.6) | 26 (76.5) | 32 (88.9) | 13 (81.3) |
| Aldosterone antagonist | 59 (68.6) | 21 (61.8) | 26 (72.2) | 12 (75.0) |
| NS beta-blocker | 36 (41.9) | 12 (35.3) | 17 (47.2) | 7 (43.8) |
| PPI | 50 (58.1) | 20 (58.8) | 22 (61.1) | 8 (50.0) |
| H2RA | 1 (1.2) | 0 (0) | 1 (2.8) | 0 (0) |
| Lactulose | 51 (59.3) | 21 (61.8) | 22 (61.1) | 8 (50.0) |
| Rifaximin | 19 (22.1) | 8 (23.5) | 8 (22.2) | 3 (18.8) |
| Laboratories | ||||
| Serum sodium, mmol/L | 133±5 | 133±5 | 133±5 | 134±5 |
| Serum creatinine, mg/dL | 1.2 (1.0–1.9) | 1.2 (1.0–2.0) | 1.2 (1.0–1.7) | 1.6 (0.7–3.6) |
| ALT, u/L | 33 (22–47) | 35 (24–47) | 34 (23–47) | 27 (17–42) |
| Serum albumin, g/dL | 2.3 (1.9–2.7) | 2.2 (1.7–2.6) | 2.4 (2.1–2.8) | 2.4 (1.9–2.9) |
| INR | 1.6 (1.4–1.8) | 1.5 (1.3–1.9) | 1.6 (1.4–1.8) | 1.6 (1.4–2.0) |
| Tbili, mg/dL | 3.1 (1.8–5.5) | 3.1 (2.0–5.5) | 3.1 (1.7–5.4) | 3.3 (1.2–5.8) |
| Platelet, cells/mm3 | 91 (61–132) | 91 (56–154) | 87 (62–124) | 107 (64–128) |
| WBC, cells/mm3 | 7.2 (5.1–10.7) | 6.8 (4.5–10.5) | 7.5 (5.0–11.0) | 8.4 (5.6–11.5) |
| Ascitic PMN, cells/mm3 | 588.6 (290.4–1977.8) | 1266.8 (275.6–3303.9) | 754.6 (470.6–1908.0) | 285.3 (190.0–646.3) |
| Ascitic total protein, g/dL | 2.72±1.17 | 2.80±0.76 | 2.65±1.61 | 2.45±0.70 |
| Liver function scores | ||||
| MELD | 16±5 | 16±5 | 16±4 | 17±5 |
| MELD-Na | 20 (16–25) | 20 (15–26) | 20 (15–24) | 19 (18–24) |
| Community acquired | 65 (75.6) | 30 (88.2) | 23 (63.9) | 12 (75.0) |
| Hospital acquired | 21 (24.4) | 4 (11.8) | 13 (36.1) | 4 (25.0) |
| Antibiotic prophylaxis | ||||
| FQ | 59 (68.6) | 24 (70.6) | 35 (97.2) | – |
| Ciprofloxacin | 56 (65.1) | 21 (61.8) | 35 (97.2) | – |
| Moxifloxacin | 3 (3.5) | 3 (8.8) | 0 (0) | – |
| TMP/SMX | 7 (8.1) | 6 (17.6) | 1 (2.8) | – |
| Cefpodoxime | 2 (2.3) | 2 (5.9) | 0 (0) | – |
| Vancomycin | 1 (1.2) | 1 (2.9) | 0 (0) | – |
| Ampicillin | 1 (1.2) | 1 (2.9) | 0 (0) | – |
Data are represented as: no. (%), median (IQR), or mean (±SD).
ACEi, angiotensin-converting-enzyme inhibitor; ALT, alanine aminotransferase; FQ, fluoroquinolone; GIB, gastrointestinal bleed; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HE, hepatic encephalopathy; HD, hemodialysis; H2RA, histamine-2 receptor antagonist; INR, international normalized ratio; MELD, Model for End Stage Liver Disease; MELD-Na, Model for End Stage Liver Disease Sodium; NS, non-selective; PMN, polymorphonuclear leukocyte count; PPI, proton pump inhibitor; Tbili, total bilirubin; TMP/SMX, trimethoprim/sulfamethoxazole; WBC, white blood cells.
Most patients were male (89.5%) with a median age of 59 years (IQR 55–64) and alcohol- (68.6%) and/or hepatitis C virus (HCV)-related (59.3%) cirrhosis. Overall, patients presented with evidence of clinically significant portal hypertension with or without decompensated cirrhosis at the time of SBP diagnosis as indicated by the presence of varices (57.0%) and hepatic encephalopathy (59.3%). More patients with daily antibiotic prophylaxis had a history of hepatocellular carcinoma (HCC) (41.2%) vs. patients receiving intermittent (25.0%) or no prophylaxis (12.5%). Medications at admission, including gastric acid suppressants, lactulose and rifaximin were similar between groups; diuretic and non-selective beta-blocker use was higher with the intermittent prophylaxis group compared with daily antibiotic prophylaxis, although non-significant (loop diuretic 88.9% vs. 76.5% [p=0.17]; aldosterone antagonist 72.2% vs. 61.8% [p=0.35]; non-selective beta-blocker 47.2% vs. 35.3% [p=0.31]). Laboratory values at the time of SBP diagnosis were similar between groups, with an overall median MELD-Na score of 20 (daily 20 [15–26] vs. intermittent 20 [15–24] vs. none 19 [18–24]).
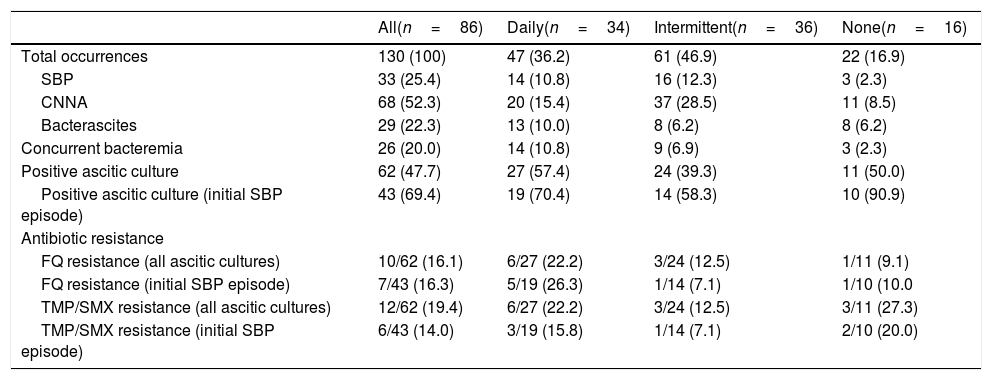
3.2SBP recurrence and mortality outcomesA total of 130 SBP episodes were analyzed from 86 patients (Table 2). Half of all episodes were CNNA (52.3%), followed by SBP (25.4%), and bacterascites (22.3%). At the first SBP episode, 75.6% of patients presented with community-acquired infection. Patients on intermittent antibiotic prophylaxis had more episodes of hospital-acquired infection (36.1%) compared to those on daily (11.8%) and no prophylaxis (25.0%).
Overall SBP characteristics.
| All(n=86) | Daily(n=34) | Intermittent(n=36) | None(n=16) | |
|---|---|---|---|---|
| Total occurrences | 130 (100) | 47 (36.2) | 61 (46.9) | 22 (16.9) |
| SBP | 33 (25.4) | 14 (10.8) | 16 (12.3) | 3 (2.3) |
| CNNA | 68 (52.3) | 20 (15.4) | 37 (28.5) | 11 (8.5) |
| Bacterascites | 29 (22.3) | 13 (10.0) | 8 (6.2) | 8 (6.2) |
| Concurrent bacteremia | 26 (20.0) | 14 (10.8) | 9 (6.9) | 3 (2.3) |
| Positive ascitic culture | 62 (47.7) | 27 (57.4) | 24 (39.3) | 11 (50.0) |
| Positive ascitic culture (initial SBP episode) | 43 (69.4) | 19 (70.4) | 14 (58.3) | 10 (90.9) |
| Antibiotic resistance | ||||
| FQ resistance (all ascitic cultures) | 10/62 (16.1) | 6/27 (22.2) | 3/24 (12.5) | 1/11 (9.1) |
| FQ resistance (initial SBP episode) | 7/43 (16.3) | 5/19 (26.3) | 1/14 (7.1) | 1/10 (10.0 |
| TMP/SMX resistance (all ascitic cultures) | 12/62 (19.4) | 6/27 (22.2) | 3/24 (12.5) | 3/11 (27.3) |
| TMP/SMX resistance (initial SBP episode) | 6/43 (14.0) | 3/19 (15.8) | 1/14 (7.1) | 2/10 (20.0) |
Data are represented as: no. (%).
CNNA, culture-negative neutrocytic ascites; FQ, fluoroquinolone; PMN, polymorphonuclear leukocyte count; SBP, spontaneous bacterial peritonitis; TMP/SMX, trimethoprim/sulfamethoxazole.
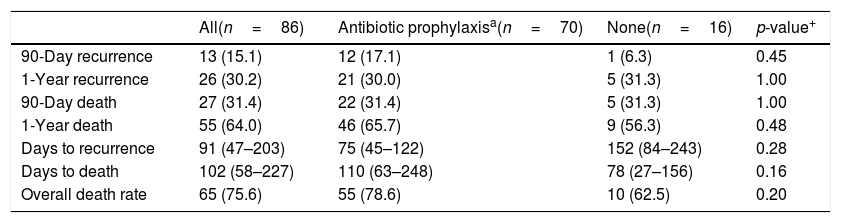
Overall 90-day and one-year mortality were 31.4% and 64.0%, respectively (Table 3). Patients receiving daily and intermittent regimens had similar rates of death at 90-days (11 [32.4%] vs. 11 [30.6%]; p=0.87) and one-year (23 [67.6%] vs. 23 [63.9%]; p=0.74) (Table 4). Patients who received no prophylaxis had similar rates of 90-day and one-year mortality to both daily and intermittent prophylaxis groups. When comparing any prophylaxis to no prophylaxis, there were no differences in 90-day (31.4% vs. 31.3%; p=1.00) or one-year mortality (65.7% vs. 56.3%; p=0.48). Common reported causes of death include comfort care (34.5%), multiorgan failure (18.2%), and end-stage liver disease (18.2%); confirmed death reports without cause of death were also observed in medical records (16.4%).
Recurrence and mortality outcomes with antibiotic prophylaxisa vs. none.
| All(n=86) | Antibiotic prophylaxisa(n=70) | None(n=16) | p-value+ | |
|---|---|---|---|---|
| 90-Day recurrence | 13 (15.1) | 12 (17.1) | 1 (6.3) | 0.45 |
| 1-Year recurrence | 26 (30.2) | 21 (30.0) | 5 (31.3) | 1.00 |
| 90-Day death | 27 (31.4) | 22 (31.4) | 5 (31.3) | 1.00 |
| 1-Year death | 55 (64.0) | 46 (65.7) | 9 (56.3) | 0.48 |
| Days to recurrence | 91 (47–203) | 75 (45–122) | 152 (84–243) | 0.28 |
| Days to death | 102 (58–227) | 110 (63–248) | 78 (27–156) | 0.16 |
| Overall death rate | 65 (75.6) | 55 (78.6) | 10 (62.5) | 0.20 |
Data are represented as: no. (%), median (IQR).
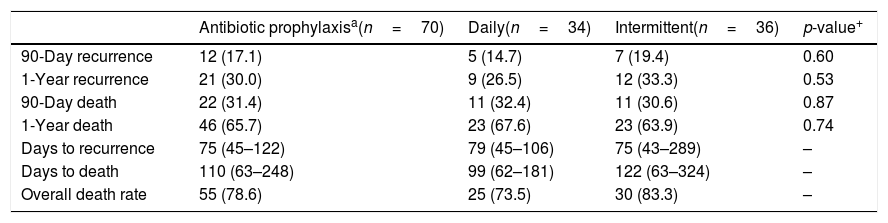
Recurrence and mortality outcomes with daily vs. intermittent prophylaxis.
| Antibiotic prophylaxisa(n=70) | Daily(n=34) | Intermittent(n=36) | p-value+ | |
|---|---|---|---|---|
| 90-Day recurrence | 12 (17.1) | 5 (14.7) | 7 (19.4) | 0.60 |
| 1-Year recurrence | 21 (30.0) | 9 (26.5) | 12 (33.3) | 0.53 |
| 90-Day death | 22 (31.4) | 11 (32.4) | 11 (30.6) | 0.87 |
| 1-Year death | 46 (65.7) | 23 (67.6) | 23 (63.9) | 0.74 |
| Days to recurrence | 75 (45–122) | 79 (45–106) | 75 (43–289) | – |
| Days to death | 110 (63–248) | 99 (62–181) | 122 (63–324) | – |
| Overall death rate | 55 (78.6) | 25 (73.5) | 30 (83.3) | – |
Data are represented as: no. (%), median (IQR).
Daily and intermittent regimens had similar rates of recurrence at 90-days (5 [14.7%] vs. 7 [19.4%]; p=0.60) and one-year (9 [26.5%] vs. 9 [33.3%]; p=0.53). There was no difference in recurrence rates between any prophylaxis vs. no prophylaxis at 90 days (17.1% vs. 6.3%; p=0.45) and one year (30.0% vs. 31.3%; p=1.00).
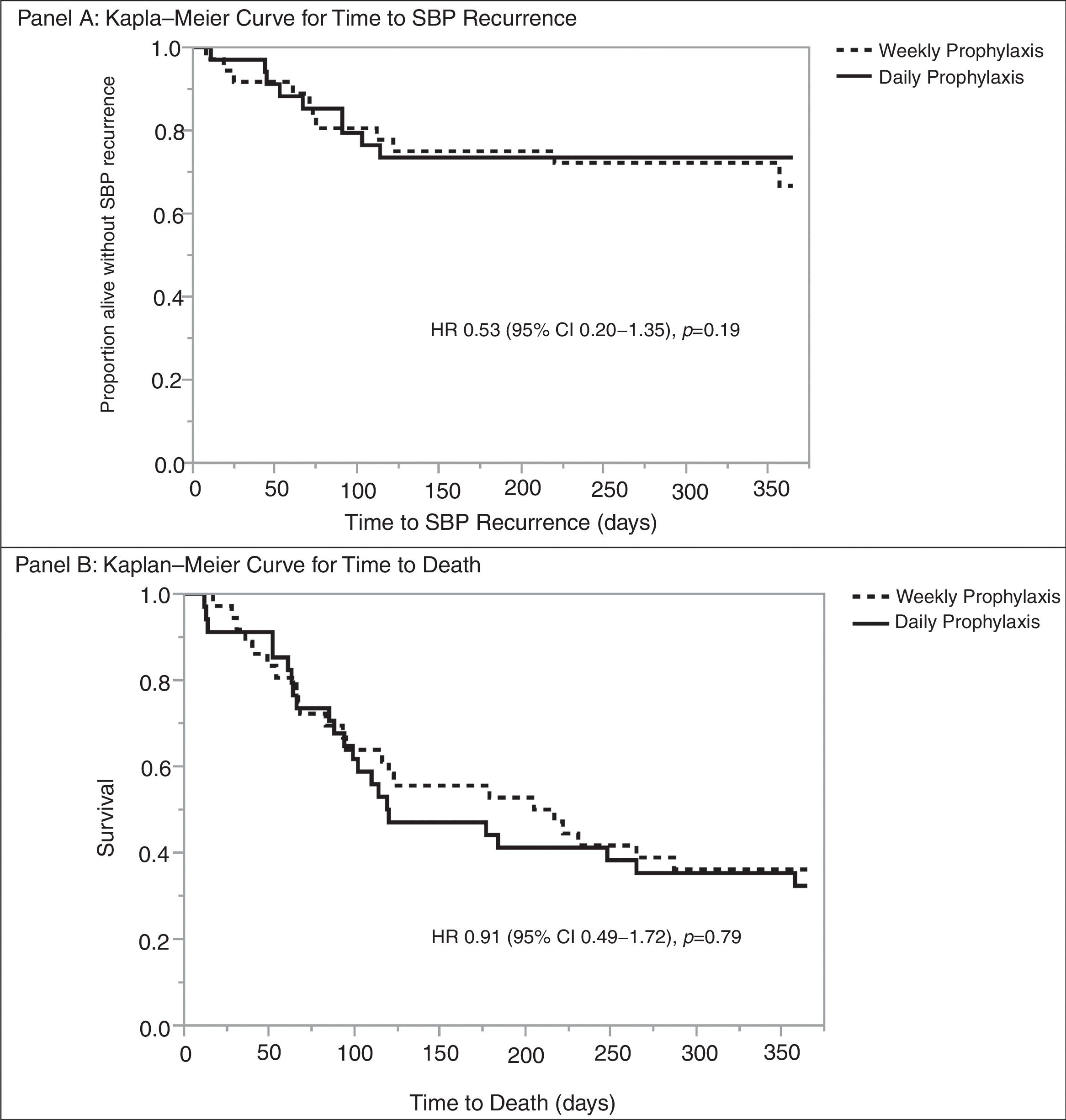
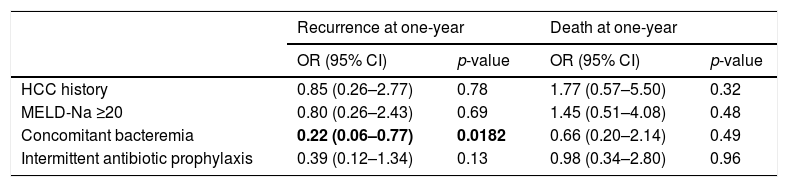
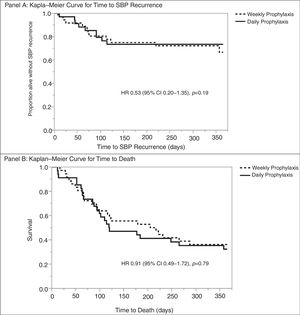
Multivariable analysis found no significant risk factors for death at one-year. Similarly, no risk factors were identified for SBP recurrence at one-year; however, concomitant bacteremia was associated with decreased rates of SBP recurrence at one year (OR 0.22 [95% CI 0.06–0.77]; p=0.0182) (Table 5). Daily vs. weekly prophylaxis did not have a significant effect on time to SBP recurrence or time to death in a Cox proportional hazards model adjusted for history of HCC, MELD-Na ≥20, or concomitant bacteremia (Fig. 2). No variables were found to be an independent predictor of time to death in the model.
Risk factors for SBP recurrence and mortality at one-year in patients receiving antibiotic prophylaxis.
| Recurrence at one-year | Death at one-year | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| HCC history | 0.85 (0.26–2.77) | 0.78 | 1.77 (0.57–5.50) | 0.32 |
| MELD-Na ≥20 | 0.80 (0.26–2.43) | 0.69 | 1.45 (0.51–4.08) | 0.48 |
| Concomitant bacteremia | 0.22 (0.06–0.77) | 0.0182 | 0.66 (0.20–2.14) | 0.49 |
| Intermittent antibiotic prophylaxis | 0.39 (0.12–1.34) | 0.13 | 0.98 (0.34–2.80) | 0.96 |
Bold entries indicate p<0.05.
*Goodness of fit for SBP recurrence at one-year, p=0.27; goodness of fit for death at one-year, p=0.17.
CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazards ratio; MELD-Na, Model for End Stage Liver Disease Sodium; OR, odds ratio; SBP, spontaneous bacterial peritonitis.
A total of eight patients (9.3%) received a liver transplant from the total cohort (median days to transplant 295 [IQR 140–1196]), of which four patients received daily prophylaxis, two patients received intermittent prophylaxis, and two received no prophylaxis.
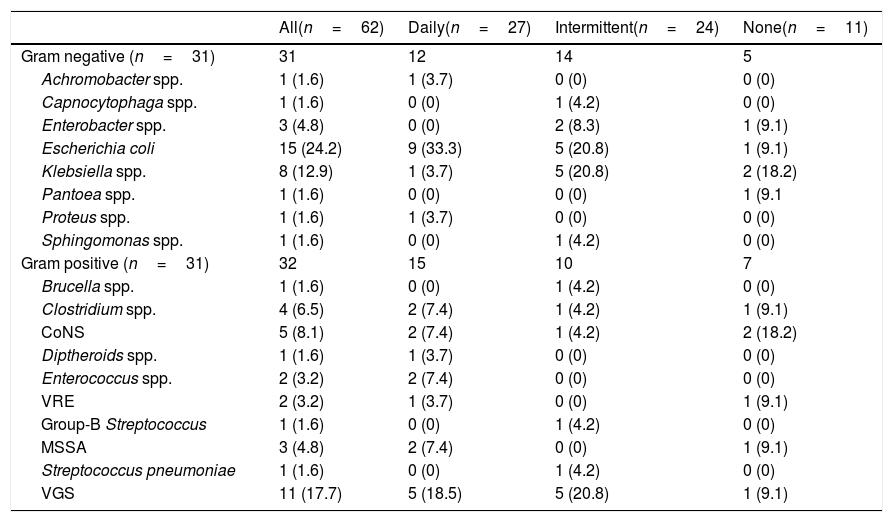
3.3Epidemiology outcomesHalf of all SBP episodes (47.7%) had positive ascitic fluid cultures. Of the 62 pathogens identified from ascitic fluid, 31 (50.0%) were gram-negative bacteria and 31 (50.0%) were gram-positive bacteria. The most common gram-negative pathogens were E. coli (48.4%) and Klebsiella spp. (25.8%). One-third of gram-positive pathogens (35.5%) were Viridans group Streptococcus (VGS) species, followed by coagulase negative Staphylococcus (CoNS) species (16.1%), and Clostridium spp. (12.9%) (Table 6).
Ascitic pathogen etiology.
| All(n=62) | Daily(n=27) | Intermittent(n=24) | None(n=11) | |
|---|---|---|---|---|
| Gram negative (n=31) | 31 | 12 | 14 | 5 |
| Achromobacter spp. | 1 (1.6) | 1 (3.7) | 0 (0) | 0 (0) |
| Capnocytophaga spp. | 1 (1.6) | 0 (0) | 1 (4.2) | 0 (0) |
| Enterobacter spp. | 3 (4.8) | 0 (0) | 2 (8.3) | 1 (9.1) |
| Escherichia coli | 15 (24.2) | 9 (33.3) | 5 (20.8) | 1 (9.1) |
| Klebsiella spp. | 8 (12.9) | 1 (3.7) | 5 (20.8) | 2 (18.2) |
| Pantoea spp. | 1 (1.6) | 0 (0) | 0 (0) | 1 (9.1 |
| Proteus spp. | 1 (1.6) | 1 (3.7) | 0 (0) | 0 (0) |
| Sphingomonas spp. | 1 (1.6) | 0 (0) | 1 (4.2) | 0 (0) |
| Gram positive (n=31) | 32 | 15 | 10 | 7 |
| Brucella spp. | 1 (1.6) | 0 (0) | 1 (4.2) | 0 (0) |
| Clostridium spp. | 4 (6.5) | 2 (7.4) | 1 (4.2) | 1 (9.1) |
| CoNS | 5 (8.1) | 2 (7.4) | 1 (4.2) | 2 (18.2) |
| Diptheroids spp. | 1 (1.6) | 1 (3.7) | 0 (0) | 0 (0) |
| Enterococcus spp. | 2 (3.2) | 2 (7.4) | 0 (0) | 0 (0) |
| VRE | 2 (3.2) | 1 (3.7) | 0 (0) | 1 (9.1) |
| Group-B Streptococcus | 1 (1.6) | 0 (0) | 1 (4.2) | 0 (0) |
| MSSA | 3 (4.8) | 2 (7.4) | 0 (0) | 1 (9.1) |
| Streptococcus pneumoniae | 1 (1.6) | 0 (0) | 1 (4.2) | 0 (0) |
| VGS | 11 (17.7) | 5 (18.5) | 5 (20.8) | 1 (9.1) |
Data are represented as: no. (%).
CoNS, coagulase negative Staphylococcus; MSSA, methicillin-susceptible Staphylococcus aureus; VGS, Viridans group Streptococcus; VRE, vancomycin-resistant Enterococcus faecium.
Twenty-six SBP episodes (20.0%) were associated with bacteremia. More patients with daily antibiotic prophylaxis had concurrent bacteremia compared to those receiving intermittent or no prophylaxis (29.8% [daily] vs. 14.8% [intermittent] vs. 13.6% [none]). E. coli was associated with the most bacteremias (26.9%), followed by S. pneumoniae (11.5%), and VGS (11.5%). Most pathogens (69.4%) were isolated from the first SBP episode, where SBP recurrences were likely to result in CNNA.
Fluoroquinolone and TMP/SMX resistance from all ascitic pathogens was 16.1% and 19.4%, respectively. Resistance to the antibiotic prophylaxis regimen was highest with E. coli, comprising 66.7% of all resistant pathogens. Of the 15 E. coli isolates cultured from ascitic fluid, 8 (53.3%) were resistant to ciprofloxacin and 7 (46.7%) were resistant to TMP/SMX.
4DiscussionComparing three study groups, daily, intermittent or no antibiotic prophylaxis, we found no significant differences in SBP recurrence or mortality at 90 days and 1 year in patients with cirrhosis and a history of SBP receiving daily, intermittent, or no antibiotic prophylaxis.
Current American and European guidelines recommend lifelong secondary antibiotic prophylaxis for all patients surviving an episode of SBP due to the high risk of SBP recurrence and mortality. Studies assessing secondary SBP prophylaxis, though now approaching 20 years, found significant decreases in recurrence of SBP as well as extraperitoneal infections compared to placebo [4–7]. Therefore, it is an unexpected finding to observe no difference in recurrence or mortality with secondary SBP antibiotic prophylaxis compared to no prophylaxis. However, current recommendations for secondary SBP prophylaxis are based on limited evidence. Studies are limited by small sample size; mixed populations comprised largely of primary SBP prophylaxis in patients with low ascitic fluid; the use of norfloxacin, an antibiotic not available in the United States; and limited follow-up ranging from one to nine months.
The preference for daily regimens over intermittent regimens is based on concerns for the development of antibiotic resistance. Data comparing daily vs. intermittent antibiotic regimens in patients with cirrhosis observed incomplete intestinal decontamination comparing fecal samples up to twelve weeks after antibiotic initiation, suggesting the superiority of daily antibiotic dosing [8]. Guidelines are limited in their recommendation for secondary SBP prophylaxis dosing strategies due to lack of robust data. Importantly, long-term comparison of daily vs. intermittent antibiotic prophylaxis for clinically significant outcomes such as recurrence and mortality has not been previously reported.
The concern for antibiotic resistance is warranted as numerous data suggest increased drug resistance with previous antibiotic exposure leading to empiric treatment failure in patients with cirrhosis with or without a history of SBP [10–14]; however, previous antibiotic exposure has not been well described. Reports of previous antibiotic exposure are limited to any systemic antibiotic exposure within a determined time [10,11], or FQ prophylaxis without a description of which agent or dosing [12–14]. In the current study, overall rates of FQ and TMP/SMX resistance were 16.1% and 19.4%, respectively, primarily driven by E. coli resistance. Almost half of E. coli isolates (n=15) were resistant to the two primary antibiotic agents reported, 8 (53.3%) were resistant to ciprofloxacin and 7 (46.7%) were resistant to TMP/SMX, which contrasts resistance patterns in our local hospital antibiogram (24% ciprofloxacin vs. 33% TMP/SMX). Interestingly, most drug-resistant E. coli isolates were cultured in the daily prophylaxis group (6/10 [daily] vs. 4/10 [intermittent] vs. 0/10 [none]). Importantly, the resistance patterns observed in the current study are contingent on a small sample size of isolates and most pathogens (69.4%) were isolated from the first SBP episode, where SBP recurrences were more likely to result in CNNA, limiting us from assessing the development antibiotic resistance. Furthermore, E. coli was the most prevalent pathogen cultured from ascitic fluid, making it difficult to compare overall resistance patterns between bacteria.
Baseline characteristics, including laboratory values and outpatient medications were similar between groups, although there are some characteristics worth discussion. Patients receiving daily prophylaxis had lower rates of diuretic use compared to intermittent prophylaxis, although non-significant (loop diuretic 76.5% vs. 88.9% [p=0.17], aldosterone antagonist 61.8% vs. 72.2% [p=0.35], respectively). Diuretics may be associated with prevention of SBP development by improving ascitic immune function in patients with low protein ascites [19], though it is unknown if differences in diuretic use in our population contributed to SBP recurrence. Non-selective beta-blocker use was also lower with daily prophylaxis compared to intermittent prophylaxis (35.5% vs. 47.2% [p=0.31], respectively). Although, contrary to diuretic use, non-selective beta-blocker use has been associated with worse outcomes, specifically increased rates of hepatorenal syndrome and death [20,21]. Patients receiving daily prophylaxis were more likely to have alcohol-related cirrhosis (79.4% vs. 55.6%) and HCC (41.2% vs. 25.0%) compared to intermittent prophylaxis. Previous literature demonstrates that patients with alcohol-related cirrhosis have shown similar or worse survival compared to HCV-related cirrhosis; HCC has been shown to be a negative prognostic factor for short-term and long-term mortality in patients with cirrhosis and ascites with and without a history of SBP [22,23]. Multivariable analysis revealed that history of HCC, MELD ≥20, and type of antibiotic prophylaxis (i.e. daily vs. intermittent prophylaxis) did not influence SBP recurrence or mortality at one year. Similarly, Cox proportional hazards model found no significant predictors of time to death. Patients on daily prophylaxis also had higher rates of bacteremia compared to intermittent prophylaxis (53.8% vs. 34.6%). Bacteremia has been independently associated with increased mortality [24]; contrary to previous reports, bacteremia had no influence on death at one year (OR 0.66 [95% CI 0.20–2.14]) and had a protective effect on SBP recurrence at one year (OR 0.22 [95% CI 0.06–0.77]). Decreased recurrence from concomitant bacteremia, while unexpected, may be a better indication of the medical treatment course. Patient's with concomitant bacteremia may have had earlier initiation of IV antibiotics and may have received longer courses of IV antibiotic therapy compared to patients with SBP alone.
Half of all pathogens identified were of gram-positive origin. This contrasts with the historical association of SBP being a gram-negative infection. Increases in gram-positive pathogens causing SBP correlates with recent reports describing similar epidemiologic changes [11–13,15–17]. Medications recommended for secondary SBP prophylaxis target gram-negative pathogens which inhabit the gastrointestinal tract. Chronic selective intestinal decontamination (SID) of gram-negative organisms disrupts gastrointestinal normal flora allowing increased presence of gram-positive organisms and drug-resistant organisms. The most commonly identified gram-positive pathogen in this cohort was VGS at 17.7% of all isolated pathogens. Similarly, Bert et al. retrospectively reviewed 84 streptococcal isolates from ascitic fluid in patients with cirrhosis during an SBP or bacterascites episode and reported 73.8% were VGS [25]. Interestingly, S. pneumoniae isolates comprised of 8.3% of all streptococcal isolates, contrary to historical data citing this pathogen as the third most frequent cause of SBP. Of note, we did not identify any methicillin-resistant Staphylococcus aureus (MRSA) isolates and overall S. aureus was a minimal cause of SBP comprising of only 4.8% of isolated pathogens in our cohort.
Our study has limitations that should be noted. Limitations of retrospective cohort studies apply to our data, including an inability to eliminate bias among groups as well as loss to follow-up. Two-thirds (70.9%) of patients received their healthcare from the Veteran's Healthcare System, which utilizes a centralized medical record database, therefore data such as medication changes and mortality were able to be captured regardless of location. We evaluated initial antibiotic prophylaxis regimens, regardless of subsequent medication changes. Four patients changed treatment regimens throughout their course; however, medication changes were generally made after a subsequent SBP recurrence while on chronic antibiotic prophylaxis. Information regarding cause of death, specifically infection-related mortality, was inconsistently reported in the medical chart. Therefore, all-cause mortality was utilized to compare study groups. Propensity score matching was performed for the intermittent and daily prophylaxis groups but resulted in a weak logistic regression model with no significant differences for any variable. Lastly, the sample size is small but similar to other published data in this clinical area.
5ConclusionIn conclusion, daily antibiotic prophylaxis was not associated with improved outcomes at 90 days or one year compared to intermittent or no antibiotic prophylaxis. Similar rates of recurrence and mortality were observed in patients receiving daily, intermittent and no prophylaxis regimens. Despite current recommendations preferring daily prophylaxis, intermittent prophylaxis is still commonly prescribed with 41.9% of the patients in our cohort on intermittent regimens. There are increasing rates of gram-positive pathogens isolated in SBP, where VGS may be an underappreciated cause. As the risk of epidemiologic changes associated with chronic SBP prophylaxis continues to be recognized, along with the finding that patients with no prophylaxis had similar outcomes to patients with any prophylaxis (either daily or intermittent), there is a need for additional evidence to evaluate the value of antibiotics in SBP prevention in the current clinical environment.AbbreviationsCNNA culture-negative neutrocytic ascites fluoroquinolone hepatitis C virus hepatocellular carcinoma Model for End-Stage Liver Disease Sodium multidrug resistant polymorphonuclear leukocyte spontaneous bacterial peritonitis trimethoprim/sulfamethoxazole
This research required no funding of any type.
Conflict of interestThe authors have no conflicts of interest to declare.
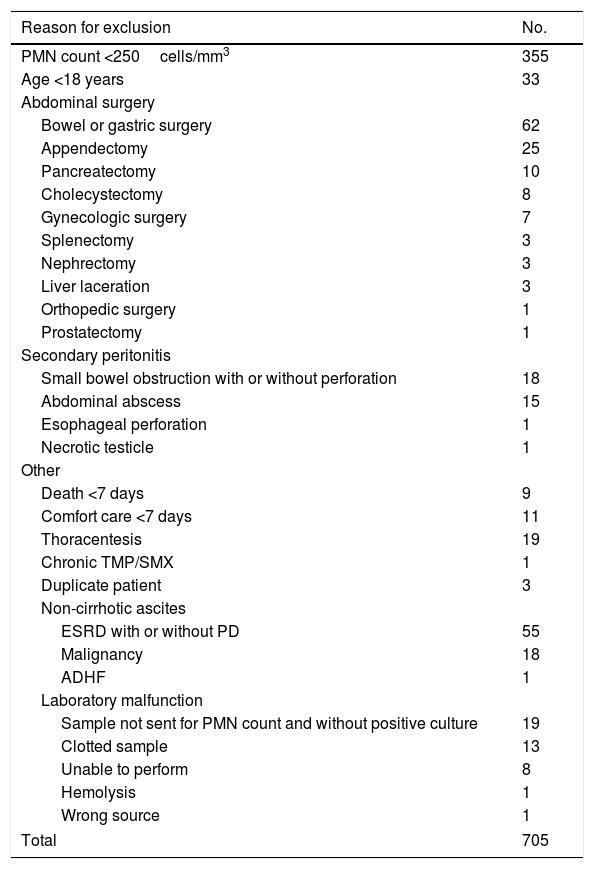
| Reason for exclusion | No. |
|---|---|
| PMN count <250cells/mm3 | 355 |
| Age <18 years | 33 |
| Abdominal surgery | |
| Bowel or gastric surgery | 62 |
| Appendectomy | 25 |
| Pancreatectomy | 10 |
| Cholecystectomy | 8 |
| Gynecologic surgery | 7 |
| Splenectomy | 3 |
| Nephrectomy | 3 |
| Liver laceration | 3 |
| Orthopedic surgery | 1 |
| Prostatectomy | 1 |
| Secondary peritonitis | |
| Small bowel obstruction with or without perforation | 18 |
| Abdominal abscess | 15 |
| Esophageal perforation | 1 |
| Necrotic testicle | 1 |
| Other | |
| Death <7 days | 9 |
| Comfort care <7 days | 11 |
| Thoracentesis | 19 |
| Chronic TMP/SMX | 1 |
| Duplicate patient | 3 |
| Non-cirrhotic ascites | |
| ESRD with or without PD | 55 |
| Malignancy | 18 |
| ADHF | 1 |
| Laboratory malfunction | |
| Sample not sent for PMN count and without positive culture | 19 |
| Clotted sample | 13 |
| Unable to perform | 8 |
| Hemolysis | 1 |
| Wrong source | 1 |
| Total | 705 |
ADHF, acute decompensated heart failure; ESRD, end-stage renal disease; PD, peritoneal dialysis; PMN, polymorphonuclear leukocyte count; TMP/SMX, trimethoprim/sulfamethoxazole.