Hepatitis B virus (HBV) surface antigen (HBsAg) to anti-HBsAg (anti-HBs) antibody seroconversion is the best, final objective for all available chronic hepatitis B (CHB) treatments. Unfortunately, this goal is rarely obtained with the currently utilized therapeutic approaches. Here we describe the case of a CHB patient who was very successfully treated with a particular therapeutic schedule. The patient was initially treated with Lamivudine for four years. Subsequently, pegylated interferon alpha-2a was introduced for a period of one year. During this period of combined therapies, the patient showed a flare of aminotransferase values followed by complete normalization of liver biochemistry parameters and HBsAg/anti-HBs seroconversion that persisted up to 24 months after all therapies had been stopped.
Hepatitis B virus (HBV) chronic hepatitis (CHB) may be a severe and progressive form of liver disease.1 Although a substantial improvement in the therapeutic treatment of CHB has been obtained in the last decade, the possibility of a complete cure of this disease is still far from being achieved since therapies available at present are incapable of eradicating the viral genomes from the liver cells.2 The currently approved anti-HBV therapies are based on the use of either Interferon-α (IFNα) 2a and 2b, or Pegylated IFN-α-2a (PEG- IFN-α-2a) or nucleoside/nucleotide analogues (NUCs) inhibiting the viral reverse transcriptase (namely, Lamivudine, Adefovir Dipivoxil, Entecavir, Telbivudine, Tenofovir).3 The main objective that these therapies can achieve is the permanent suppression of viral replication and above all-the HBV surface antigen (HBsAg) seroclearence with the appearance of the corresponding antibody (anti-HBs). However, this HBsAg/anti-HBs seroconversion can be obtained only in few IFN-treated cases and even more rarely in patients treated with NUCs.3 Since IFN and NUCs have different and theoretically complementary modes of action, a therapeutic approach combining both drugs might hypothetically be a good option for the treatment of CHB.4-13 However, this combination therapy has been insufficiently investigated so far, and in particular no valid information is available on possible therapeutic schedules foreseeing different timing in the administration of the IFN and NUC. Here we report the case of a CHB patient showing a very favourable clinical and virological outcome by means of treatment with Lamivudine and subsequent PEG-IFN-α-2a add-on.
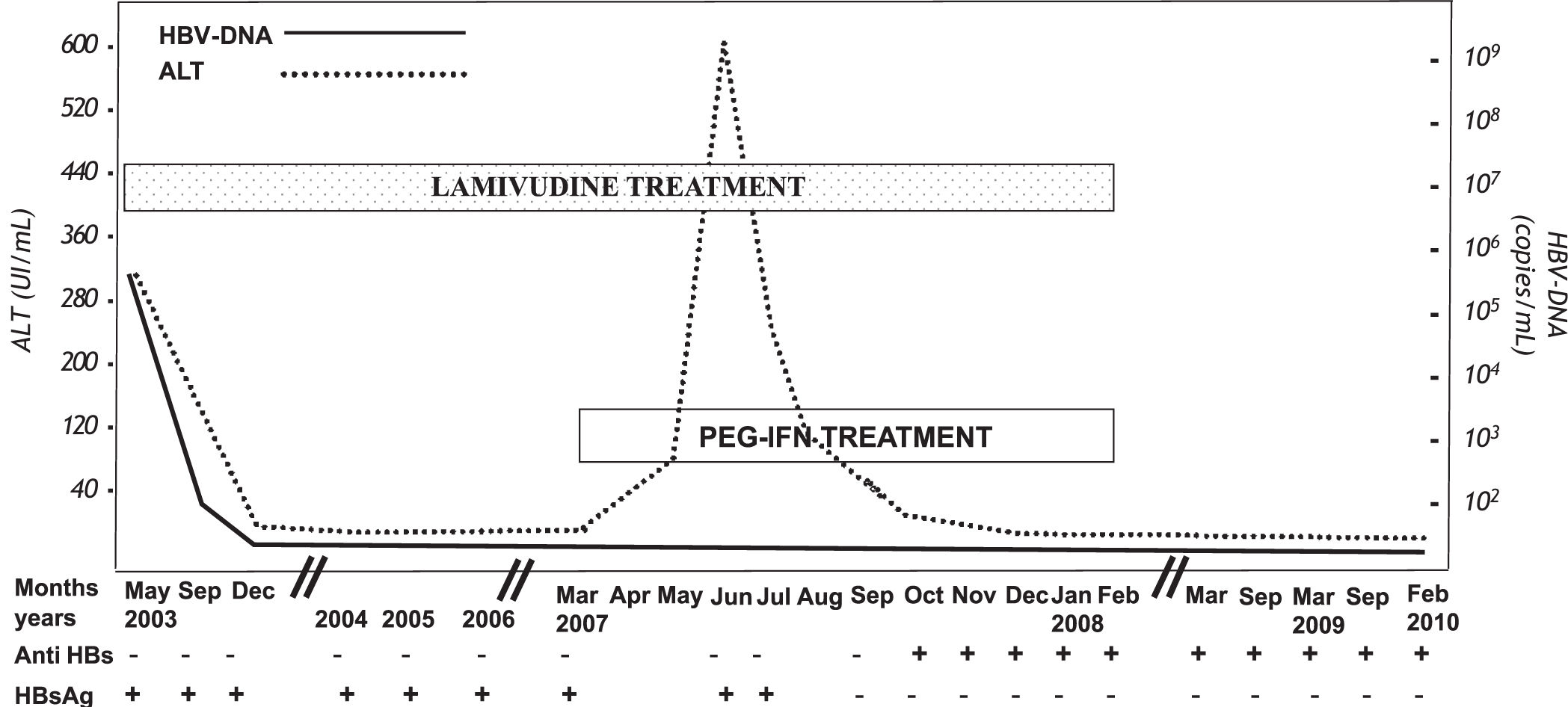
Case ReportA 20 year old Italian male was admitted to the outpatients clinic of the Infectious Diseases Division of the “Bianchi-Melacrino-Morelli Hospital” of Reggio Calabria (Italy) in March 2003 because of a previously diagnosed –but never treated– CHB. He denied familial history of HBV infection as well as major risk factors for viral infection. He declared that he had occasionally been found to be HBsAg positive in infancy and to have had fluctuating values of aminotransferase values over time. A needle liver biopsy performed in another hospital in 2002 had shown a mild degree of chronic hepatitis [(G5/F1, according to Knodel score)].14 At clinical observation the patient presented with normal body mass index, mild hepatomegaly in the absence of splenomegaly. He denied a history of drug or alcohol abuse, there was no evidence of other known causes of liver disease, and he tested negative for hepatitis Delta, hepatitis C and immunodeficiency virus serum markers. The alanine aminotransferase (ALT) levels were twice the upper normal levels (UNL), the HBV profile showed the “e” antigen to be negative and the corresponding antibody to be positive and the viral DNA was 17,600 copies/mL (Amplicore HBV Monitor kit, Roche Diagnostics). At this time the patient was not treated. Two months later (May 2003) he had a hepatitis flair with ALT levels 8 times the UNL and 450,000 copies/mL of HBV DNA. At this point, treatment with Lamivudine 100 mg/day was started (Figure 1). The patient -who attended all the scheduled follow-up visits-had a prompt virological/biochemical response, achieving normal ALT values and undetectable levels of HBV-DNA in six months. At the following visits he always showed normal ALT levels together with undetectable HBV DNA but with the persistence of HBsAg. At the clinical check-up in March 2007 (46 months after the start of therapy), the patient expressed several doubts about taking Lamivudine every day for the rest of his life and his intention to discontinue the treatment soon was clear. Considering the high probability of HBV reactivation in the event of discontinuing Lamivudine treatment and, on the other hand, the fact that the best chances of success of IFN therapy in CHB appear to occur when the viremia levels are low, we proposed a therapeutic schedule based on the maintenance of Lamivudine and the add-on of PEG-IFN-α-2a for a period of 12 months. Our hope was that this regimen could help to permanently transform the HBV infection from active to inactive and, consequently, to avoid the reactivation of the disease once the patient discontinued the Lamivudine therapy. The patient accepted this proposal, providing his informed consent. Therefore, in March 2007 we added PEG-IFN-α-2a 180 μg/week to the pre-existing Lamivudine administration for a subsequent period of 12 months. During the first weeks of the combined therapy, the patient suffered mild, typical IFN side effects such as fever and fatigue, whereas the liver function tests were persistently normal with undetectable HBV DNA. However, at the clinical check-up performed 8 weeks after starting PEG- IFN-α-2a therapy a three fold UNL increase of ALT was observed, with HBV DNA values still undetectable. One month later (12 weeks after staring PEG-IFN-α-2a therapy) a flair of aminotransferase (ALT up to 15 times the UNL and aspartate aminotransferase up to 8 times UNL) was detected. All known causes of liver damage were excluded, including super-infection with other hepatotropic viruses and toxics. Considering that the patient had no subjective symptoms, the bilirubin, prothrombin and albumin values were normal and HBV DNA was persistently undetectable, the combined treatment was continued despite the flair and more frequent clinical check-ups were established. The aminotransferase levels progressively decreased and definitively returned to normal values in December 2007. Very surprisingly, at the October 2007 check-up the HBsAg tested negative and one month later the anti-HBs became positive. At the end of one year of Lamivudine + PEG-IFN-α-2a treatment (February 2008), the patient had normal liver biochemistry and undetectable HBV-DNA and he was HBsAg-negative and anti-HBs positive, maintaining this status of complete biochemical/virological recovery at the check-ups performed 6, 12, 18 and 24 months after stopping all treatment (Figure 1).
DiscussionHBsAg/anti-HBs seroconversion marks the clinical recovery of CHB and, consequently, it represents the main objective of any therapy. Unfortunately, however, the treatments available at present rarely allow this objective to be achieved. In the case reported here, the HBsAg/anti-HBs seroconversion was achieved by the use of a particular therapeutic schedule where PEG-IFN-α-2a was added-on in a patient who apparently had an optimal response to La-mivudine administration with persistent suppression of HBV replication and lack of emergence of any viral strain resistant to this particular NUC. Intriguingly, the seroconversion occurred after a flare of aminotransferase values, an event that is difficult to explain. In fact, the top level of the flare was observed after three months of combination therapy and appeared to be independent of a reactivation of the HBV infection since the frequent virological follow up performed under treatment showed persistently undetectable levels of serum HBV DNA. One might explain the flare with the toxic effect that PEG-IFN may exert on liver tissue,15,16 although it has to be stressed that our patient experienced a much more pronounced ALT increase (598 IU/mL) than that usually associated with PEG-IFN therapy, and -more importantlyliver biochemistry slowly returned under the normal value levels even though the PEG-IFN-α-2a administration was not interrupted. Considering that liver cell expression of the HBV proteins may persist independently of NUC-induced suppression of viral replication, we might hypothesize that the ALT flare and subsequent HB-sAg seroclearance observed in our patient might be related to the lysis of hepatocytes expressing HBV antigens and due to the restoration of an efficient anti-HBV immunoresponse induced by the IFN treatment.1718 Combination therapy with PEG-IFN and NUCs is an understudied approach for the cure of CHB. In fact, there is no reliable information concerning which NUC is best to combine with IFN, the timing in giving each drug and the duration of their administration. The excellent outcome of our case may encourage the design of the large scale clinical trials necessary to definitively clarify the usefulness and the best schedule of this combination therapy.