Introduction and aim. Spontaneous bacterial peritonitis (SBP) is a life-threatening infection in patients with cirrhosis. However, it is unknown whether patients with SBP and cirrhosis who do not have active gastrointestinal bleeding have a poorer prognosis if treated with proton pump inhibitors (PPI).
Material and methods. We used the Taiwan National Health Insurance Database to identify 858 patients with SBP and cirrhosis who were administered PPIs and hospitalized between January 1, 2010, and December 31, 2013. One-to-two propensity score matching was performed to select a comparison group based on age, gender, and comorbidities. All patients obtained follow-up for 1 year.
Results: The overall 30-day, 90-day, and 1-year mortality was 27.9%, 49.0%, and 73.7%, respectively, in the PPI group and 25.6%, 43.8%, and 67.2%, respectively, in the non-PPI group. After adjusting the Cox regression model for age, gender, and comorbidities, the hazard ratios for PPIs regarding 30-day, 30- to 90-day, and 90-day to 1-year mortality were 1.074 (95% CI 0.917-1.257, P = 0.377), 1.390 (95% CI 1.154-1.673, P = 0.001), and 1.297 (95% CI 1.0991.531, P = 0.002), respectively.
Conclusions: PPIs did not increase the short-term mortality of patients with SBP and cirrhosis who did not have active gastrointestinal bleeding, but PPIs increased the long-term mortality risk. For these patients, physicians should discontinue PPIs as early as possible.
Cirrhosis is a hepatic disorder characterized by hepatic cell death, inflammation, and fibrotic conversion of the liver, thus increasing susceptibility to bacterial infection.1 If patients with cirrhosis have bacterial infections, they have a four-fold increase in mortality.2 Spontaneous bacterial peritonitis (SBP) is a specific life-threatening infection in patients with cirrhosis.1–5 It is caused by invasive procedures or bacterial translocation from the intestine because of bowel bacterial overgrowth, increased intestinal permeability, and impaired immunity in patients with cirrhosis.1–10
A proton pump inhibitor (PPI) is a kind of potent acid suppressant used for gastroesophageal reflux disease and peptic ulcers.11 In patients with cirrhosis, PPIs can reduce the size of postbanding ulcers after endoscopic variceal ligation.12 Some reports have indicated that 46%-78% of patients with cirrhosis use PPIs.13,14 However, recent reports have indicated that PPIs can facilitate intestinal bacterial overgrowth and increase bacterial translocation of the intestine, which increases the occurrence of SBP in patients with cirrhosis.15–19 Studies in mice have also demonstrated a high colonization rate with vancomycin-resistant Entero-coccusfaecium or drug-resistant Klebsiella pneumoniae in those administered PPIs.20 In some studies, PPIs increased the mortality of patients with cirrhosis.14,21
However, patients with cirrhosis using PPIs often have active gastrointestinal bleeding or recently had gastrointestinal bleeding and are prone to higher mortality. It is unknown if the increased mortality risk is attributable to PPIs or gastrointestinal bleeding. To eliminate this bias in the present study, we enrolled only patients with SBP and cirrhosis who did not have active gastrointestinal bleeding. Using the Taiwan National Health Insurance Research Database, we enrolled a large population of patients with SBP and cirrhosis who did not have active gastrointestinal bleeding and identified the effect of PPIs on their mortality.
Material and MethodsDatabase and ethical statementThe Taiwanese government started the National Health Insurance program in 1995. Currently, the National Health Insurance Bureau covers more than 98% of the Taiwanese population. All contracted medical institutions must provide medical records to the National Health Insurance Bureau for medical payment. The National Health Insurance Bureau and National Health Research Institute used these medical records to establish a database called the National Health Insurance Research Database. Investigators can use a dataset from the National Health Insurance Research Database in their studies, but the study protocols must be evaluated and approved by the National Health Research Institute.
This study used a dataset from the National Health Insurance Research Database that contained all International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for hospitalized patients in Taiwan. The agreement number was 104359. This study also obtained the approval of the institutional review board of the Buddhist Dalin Tzu Chi Hospital (IRB B10403026). The review board waived the requirement for written informed consent from all patients because all identifying personal information in the dataset was removed before analysis.
Study sampleWe screened for patients who were discharged with a primary or an accessory diagnosis of cirrhosis (ICD-9-CM code 571.5, or 571.2) between January 1, 2010, and December 31, 2013. SBP was defined as patients with ICD-9-CM codes 567.2, 567.8, or 567.9. Patients were not included if they had another diagnostic code for secondary peritonitis such as appendicitis, hollow organ, or biliary tract perforation; ischemic bowel disease; or peritoneal dialysis catheter-related peritonitis; or had an additional procedure code for abdominal surgery.3,4,22 If patients had multiple hospitalizations for SBP, only the first SBP episode was selected for analysis. To exclude patients with cirrhosis who had active gastrointestinal bleeding, patients were excluded if they had diagnostic codes for upper gastrointestinal tract bleeding (ICD-9-CM code 531.0, 531.2, 531.4, 531.6, 532.0, 532.2, 532.4, 532.6, 533.0, 533.2, 533.4, and 533.6) or esophageal variceal bleeding (ICD-9-CM code 456.0 or 456.0). In addition, those undergoing panendoscopy or administered an intravenous PPI during hospitalization were also excluded. Because high-dose oral PPIs might be used to treat recent peptic ulcer bleeding, we also excluded those using PPIs at higher than standard doses (standard doses: omeprazole 20 mg, rabeprazole 20 mg, lansoprazole 30 mg, pantoprazole 40 mg, and esomeprazole 40 mg).
As for the enrolled patients, those who were administered oral PPIs including esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole were considered the study group (PPI group). To avoid interference from any of the measured confounding factors, one-to-two propensity score matching was performed to select the control group (non-PPI group) according to comorbidities, including age, gender, alcoholism (ICD-9-CM codes 291, 303, 305.00-305.03, and 571.0-571.3), hepatocellular carcinoma (ICD-9-CM code 155.0), hepatic encephalopathy (ICD-9-CM code 572.2), and renal function impairment (ICD-9-CM code 584, 585, 586, and 572.4, as well as any other procedural codes related to renal failure).
Statistical analysesWe used the SPSS statistical package (SPSS System for Windows, version 22.0) to perform the analyses in this study. A χ2 test or Fisher’s exact test was used to compare categorical variables. A Student’s t-test was used to compare continuous variables. To identify risk factors for mortality in patients with SBP and cirrhosis, a Cox regression model (proportional hazards model) was used to control the effects of confounding factors. Hazard ratios (HR) are presented along with a 95% CI using a significance level of 0.05.
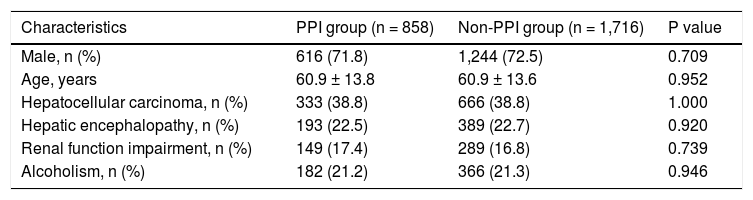
ResultsA total of 13,278 patients with SBP and cirrhosis were contained in the dataset. Of all, 11,982 cirrhotic patients could be followed for 1 year. After excluding those with diagnostic codes for a peptic ulcer, esophageal variceal bleeding, or panendoscopy or who were administered intravenous PPIs therapy or a higher dose of oral PPIs, this study enrolled a total of 6,452 patients with SBP and cirrhosis, but without active gastrointestinal bleeding. There were 858 patients who were administered oral PPIs during their hospitalization and were considered the PPI group. After 1:2 propensity score matching, the comparison group consisted of 1,716 patients with SBP and cirrhosis who were not administered oral PPIs and were considered the non-PPI group. In the PPI group, the mean age was 60.9 ± 13.8 years, and 616 (71.8%) patients were males (Table 1).
Demographic characteristics of patients with SBP and cirrhosis who did or did not concomitantly use oral PPIs.
| Characteristics | PPI group (n = 858) | Non-PPI group (n = 1,716) | P value |
|---|---|---|---|
| Male, n (%) | 616 (71.8) | 1,244 (72.5) | 0.709 |
| Age, years | 60.9 ± 13.8 | 60.9 ± 13.6 | 0.952 |
| Hepatocellular carcinoma, n (%) | 333 (38.8) | 666 (38.8) | 1.000 |
| Hepatic encephalopathy, n (%) | 193 (22.5) | 389 (22.7) | 0.920 |
| Renal function impairment, n (%) | 149 (17.4) | 289 (16.8) | 0.739 |
| Alcoholism, n (%) | 182 (21.2) | 366 (21.3) | 0.946 |
PPI: proton pump inhibitor.
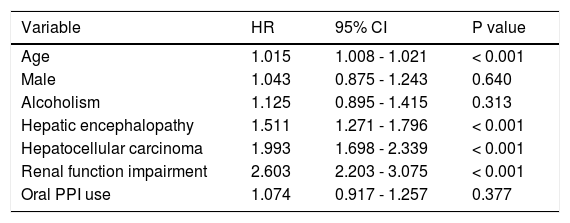
The overall 30-day mortality for the PPI group and non-PPI group was 27.9% and 25.6%, respectively. After adjusting the Cox regression model for age, gender, and other comorbidities, the HR for oral PPIs regarding the 30-day mortality for patients with SBP and cirrhosis who did not have an active gastrointestinal bleed was 1.074 (95% CI 0.917-1.257, P = 0.377), as compared with the non-PPI group. The other significantly different prognostic factors were as follows: hepatocellular carcinoma (HR 1.993, 95% CI 1.698-2.339, P < 0.001), hepatic encephalopathy (HR 1.511, 95% CI 1.271-1.796, P < 0.001), renal function impairment (HR 2.603, 95% CI 2.203-3.075, P < 0.001), and age (HR 1.015, 95% CI 1.008-1.021, P < 0.001) (Table 2).
Adjusted HRs for risk factors regarding the 30-day mortality of patients with SBP and cirrhosis and without active gastrointestinal bleeding.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.015 | 1.008 - 1.021 | < 0.001 |
| Male | 1.043 | 0.875 - 1.243 | 0.640 |
| Alcoholism | 1.125 | 0.895 - 1.415 | 0.313 |
| Hepatic encephalopathy | 1.511 | 1.271 - 1.796 | < 0.001 |
| Hepatocellular carcinoma | 1.993 | 1.698 - 2.339 | < 0.001 |
| Renal function impairment | 2.603 | 2.203 - 3.075 | < 0.001 |
| Oral PPI use | 1.074 | 0.917 - 1.257 | 0.377 |
CI: confidence interval. HR: hazard ratio. PPI: proton pump inhibitor.
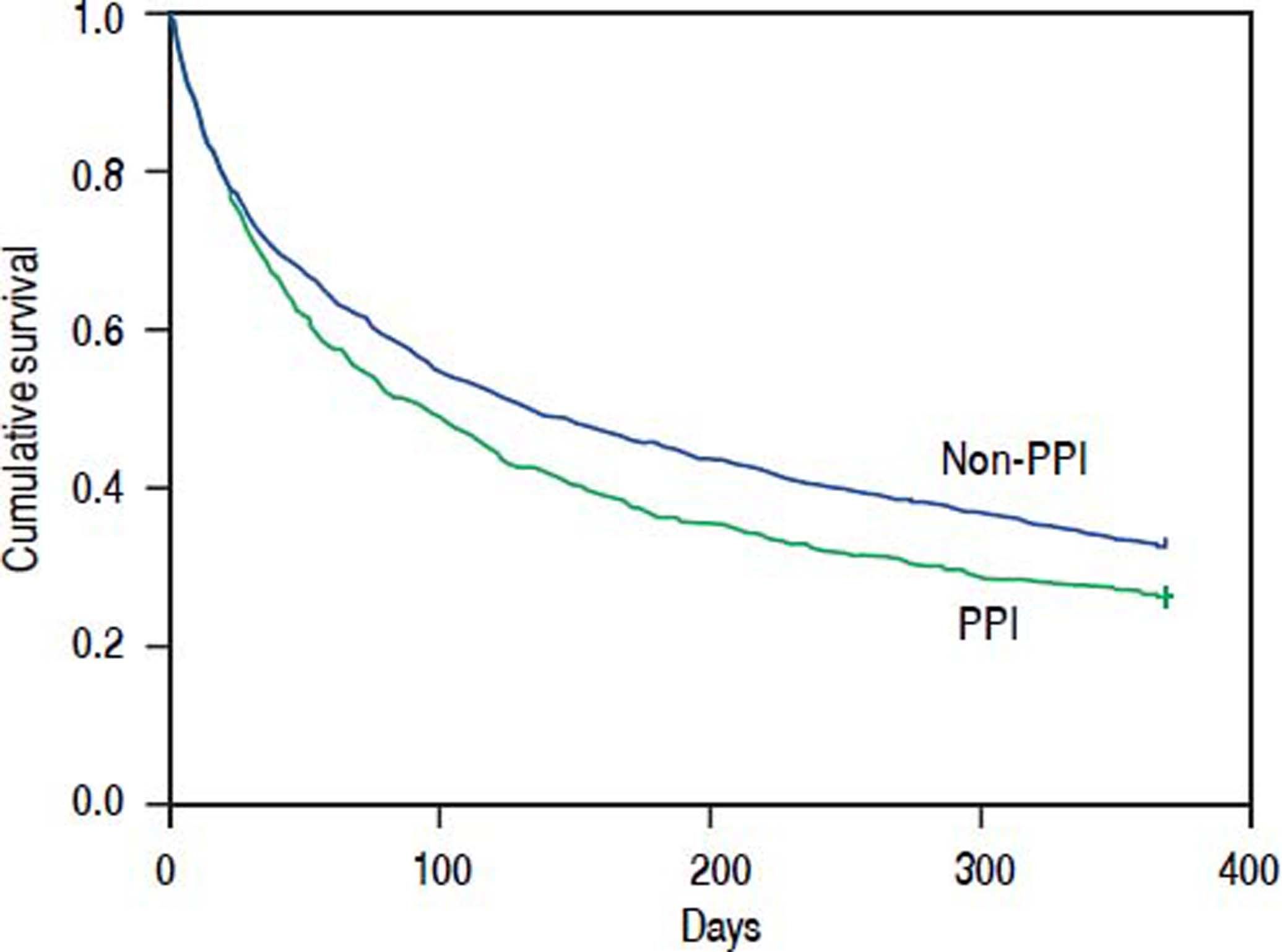
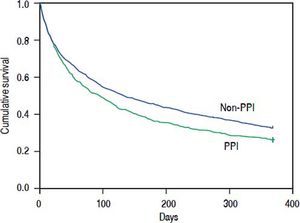
To evaluate mortality occurring at a later time, we calculated the 90-day mortality for the patients who survived more than 30 days and the 1-year mortality for the patients who survived more than 90 days. The 90-day and 1-year mortality was 49.0%, and 73.7%, respectively, in the PPI group, and 43.8%, and 67.2%, respectively, in the non-PPI group (Figure 1). After the Cox regression model was adjusted for age, gender, and other comorbidities, the HRs for oral PPIs regarding the 30- to 90-day and 90-day to 1-year mortality were 1.390 (95% CI 1.154-1.673, P = 0.001) and 1.297 (95% CI 1.099-1.531, P = 0.002), respectively (Table 3).
Adjusted HRs for different PPIs regarding the 30-day, 30- to 90-day, and 90-day to 1-year mortality of patients with SBP and cirrhosis and without active gastrointestinal bleeding
| PPIs | 30-day mortality | 30- to 90-day mortality | 90-dday to 1-year mortality | |||
|---|---|---|---|---|---|---|
| Case/control | HR (95% CI) | Case/Control | HR (95% CI) | Case/Control | HR (95% CI) | |
| PPIs | 858/1716 | 1.074 (0.917-1.257) | 619/1276 | 1.390 (1.154-1.673) | 438/981 | 1.297 (1.099-1.531) |
| Esomeprazole | 286/1716 | 1.226 (0.978-1.537) | 195/1276 | 1.568 (1.198-2.053) | 130/981 | 1.447 (1.119-1.872) |
| Lansoprazole | 235/1716 | 1.248 (0.979-1.591) | 158/1276 | 1.474 (1.082-2.008) | 111 / 981 | 1.328 (0.996-1.770) |
| Omeprazole | 24/1716 | 0.486 (0.156-1.513) | 21/1276 | 1.225 (0.505-2.973) | 16 / 981 | 0.767 (0.341-1.723) |
| Pantoprazole | 211/1716 | 0.671 (0.483-0.931) | 172/1276 | 1.226 (0.898-1.674) | 126/981 | 1.255 (0.958-1.643) |
| Rabeprazole | 68/1716 | 1.330 (0.848-2.085) | 48/1276 | 0.798 (0.395-1.613) | 40/981 | 1.191 (0.743-1.912) |
CI: confidence interval. HR: hazard ratio. PPI: proton pump inhibitor.
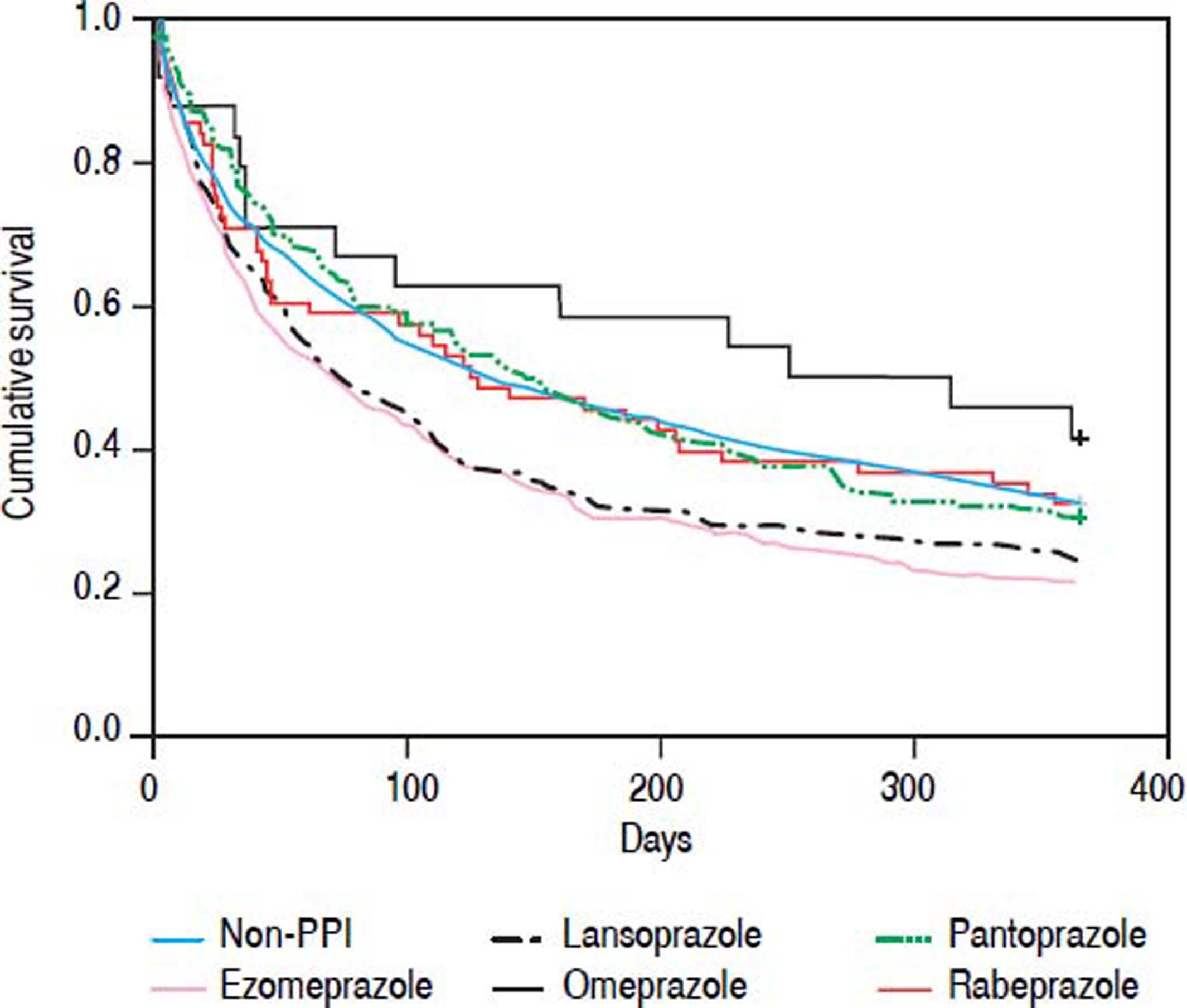
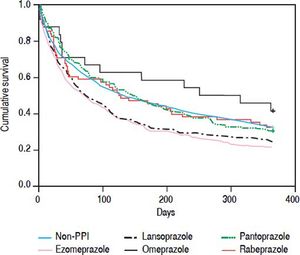
Each of the oral PPIs was compared with the non-PPI group to evaluate the effect of each oral PPI on the mortality of patients with SBP and cirrhosis, but without active gastrointestinal bleeding. Patients receiving only one kind of oral PPI during their hospitalization were enrolled. Oral pantoprazole (HR 0.671, 95% Cl 0.483-0.931, P = 0.017) showed a beneficial effect on the 30-day mortality of patients with SBP and cirrhosis who did not have an active gastrointestinal bleed. However, other oral PPIs had no effect on the 30-day mortality. Oral esomeprazole (HR 1.568, 95% Cl 1.198-2.053, P = 0.001) and lansoprazole (HR 1.474, 95% CI 1.082-2.008, P = 0.014) had a higher 30 to 90-day mortality risk. Oral esomeprazole (HR 1.447, 95% CI 1.119-1.872, P = 0.005) had a higher 90-day to 1-year mortality risk, but the other PPIs had no effect (Table 3; Figure 2).
DiscussionThis was a nationwide population-based study conducted to identify the effect of oral PPIs on the outcomes of patients with SBP and cirrhosis who had no active gastrointestinal bleeding. Using the national population-based database to enroll a large population of patients with SBP and cirrhosis, our study provides reliable information and reflects the actual mortality in clinical practice. Our study showed SBP contributed to a poor prognosis in patients with cirrhosis. The 30-day mortality was over 20%, and the 1-year mortality was over 60%. This result is comparable to a previous study involving patients with SBP and cirrhosis.2–4,10,21–24
Patients with cirrhosis commonly have gastrointestinal bleeding including esophageal variceal bleeding, peptic ulcer bleeding, portal hypertensive gastropathy, and gastric vascular ectasia syndrome. In such patients, PPIs are used for the treatment or prevention of bleeding after endoscopic hemostasis.12,25 However, recent studies showed that PPIs could increase the occurrence of SBP in patients with cirrhosis.15–19 In a retrospective case-control study, there were 25 patients with SBP and cirrhosis who were administered PPIs and 26 patients who were not administered PPIs. Patients with and without PPI use responded equally well to antibiotics (84% vs. 81%, respectively, P = 0.8) and experienced no significant difference in mortality (84% vs. 73%, respectively, P = 0.3).26 In another retrospective study, there were 82 patients with SBP and cirrhosis who were administered PPIs and 451 who were not administered PPIs. The result showed that the use of PPIs was a predictor of 30-day mortality (HR 1.960, 95% CI 1.190-3.227, P = 0.008).25 The former study was limited by a small case number. The latter study had a larger study population, but the PPI group had more patients with hepatocellular carcinoma and esophageal variceal bleeding. We could not know if a patient was administered PPIs continuously or had active gastrointestinal bleeding during hospitalization. Patients with cirrhosis who had a history of esophageal variceal bleeding are more prone to receiving PPIs continuously and having esophageal variceal bleeding during hospitalization for SBP.27 The higher mortality risk may be attributed to active gastrointestinal bleeding during hospitalization for SBP, but not to the PPI.
To eliminate this confounding factor, we excluded patients with SBP and cirrhosis who had active gastrointestinal bleeding during hospitalization. We used a 1:2 propensity score match to select a comparison group to control for other comorbidities. We found that concomitant oral PPI did not increase 30-day mortality risk in patients with SBP and cirrhosis who did not have active gastrointestinal bleeding. A recent study showed that PPIs could inhibit the production of proinflammatory cytokines by monocytes and protect against lipopolysaccha-ride-induced mortality in a murine model of lethal endotoxic shock.28 However, the present study did not demonstrate that PPIs had this benefit in patients with SBP and cirrhosis.
In our study, PPI users had a higher 30- to 90-day and 90-day to 1-year mortality than non-PPI users. This may be attributed to the increased occurrence of SBP in patients with cirrhosis who used PPIs. We suggest that oral PPIs should be discontinued in patients with SBP and cirrhosis who do not have active gastrointestinal bleeding as soon as possible. However, patients with cirrhosis who have peptic ulcer diseases still need to take oral PPIs to prevent recurrent bleeding and promote ulcer healing. A question still remains as to how long oral PPIs should be used in patients with cirrhosis who have peptic ulcer disease for the greatest survival benefit.
The present study showed that pantoprazole had a beneficial effect on 30-day mortality but that esomeprazole had a harmful impact on 30- to 90-day and 90-day to 1-year mortality in patients with SBP and cirrhosis, but without active gastrointestinal bleeding. Esomeprazole was more effective for the relief of acid reflux symptoms in patients with reflux esophagitis than other PPIs.29,30 Based on pharmacokinetic studies, acid inhibition is faster and superior with esomeprazole than pantoprazole, and esomeprazole is more effective for controlling gastric acid at a steady state than other PPIs.31,32 According to the present study, the PPI with more effective gastric acid control seems to carry a higher mortality risk in patients with SBP and cirrhosis and without active gastrointestinal bleeding. However, further study is necessitated to demonstrate this observation.
There were several limitations in our study. First, we could not evaluate the severity of cirrhosis through the Mayo Clinic model for end-stage liver disease score or Child-Pugh score. This is because the dataset that was used in this study did not include laboratory data such as bilirubin levels, albumin levels, or prothrombin time. Second, the exact etiology of non-alcoholic cirrhosis could not be identified. This is because the cause of cirrhosis was not always coded in patients’ hospitalizations. However, previous studies have established that the etiology of non-alcohol-related cirrhosis in Taiwan is primarily associated with the hepatitis B virus.33 Third, the duration of PPI exposure was not available before admission for SBP. However, it has been demonstrated that PPI use 48 h after the initial dose induces a sustained increase in gastric acid suppression.34 Fourth, we could not know exactly how long the patient received PPIs after discharge. Therefore, we could not identify the effect of the duration of oral PPIs on the mortality of patients with cirrhosis. Finally, this was a retrospective study with selection bias. However, we used propensity score matching and a Cox regression model to reduce this bias as much as possible. Despite these limitations, this study still provided some useful clinical information for clinical practice. In conclusion, this nationwide population-based study showed that concomitant oral PPI use did not increase the 30-day mortality of patients with SBP and cirrhosis who did not have active gastrointestinal bleeding. However, PPI users had a higher 30- to 90-day and 90-day to 1-year mortality than non-PPI users. We recommend that physicians discontinue PPIs as soon as possible in patients with SBP and cirrhosis who do not have active gastrointestinal bleeding.
Abbreviations- •
HR: hazard ratio.
- •
ICD-9-CM: International Classification of Diseases, 9th Revision, Clinical Modification.
- •
PPI: proton pump inhibitor.
- •
SBP: spontaneous bacterial peritonitis.
The authors declares that there is no conflict of interest regarding the publication of this article.
AcknowledgmentsThis study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and managed by the National Health Research Institutes (Registered number: 104359). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health or National Health Research Institutes.
Compliance With Ethical RequirementsFor studies with human subjects, this study was initiated after approval by the institutional review board of the Buddhist Dalin Tzu Chi General Hospital Taiwan (B10403026). As all identifying personal information was stripped from the secondary files before analysis, the review board waived the requirement for written informed consent from the patients involved.