Direct antiviral agents (DAAs) including sofosbuvir (SOF), daclatasvir (DCV), simeprevir (SIM) and ombitasvir, paritaprevir and dasabuvir were introduced 2015 in Brazil for treatment of hepatitis C virus (HCV) infection. The aims of this study were to assess effectiveness and safety of HCV treatment with DAA in real-life world in a highly admixed population from Brazil.
Materials and methodsAll Brazilian reference centers for HCV treatment were invited to take part in a web-based registry, prospectively conducted by the Brazilian Society of Hepatology, to assess outcomes of HCV treatment in Brazil with DAAs. Data to be collected included demographics, disease severity and comorbidities, genotype (GT), viral load, DAA regimens, treatment side effects and sustained virological response (SVR).
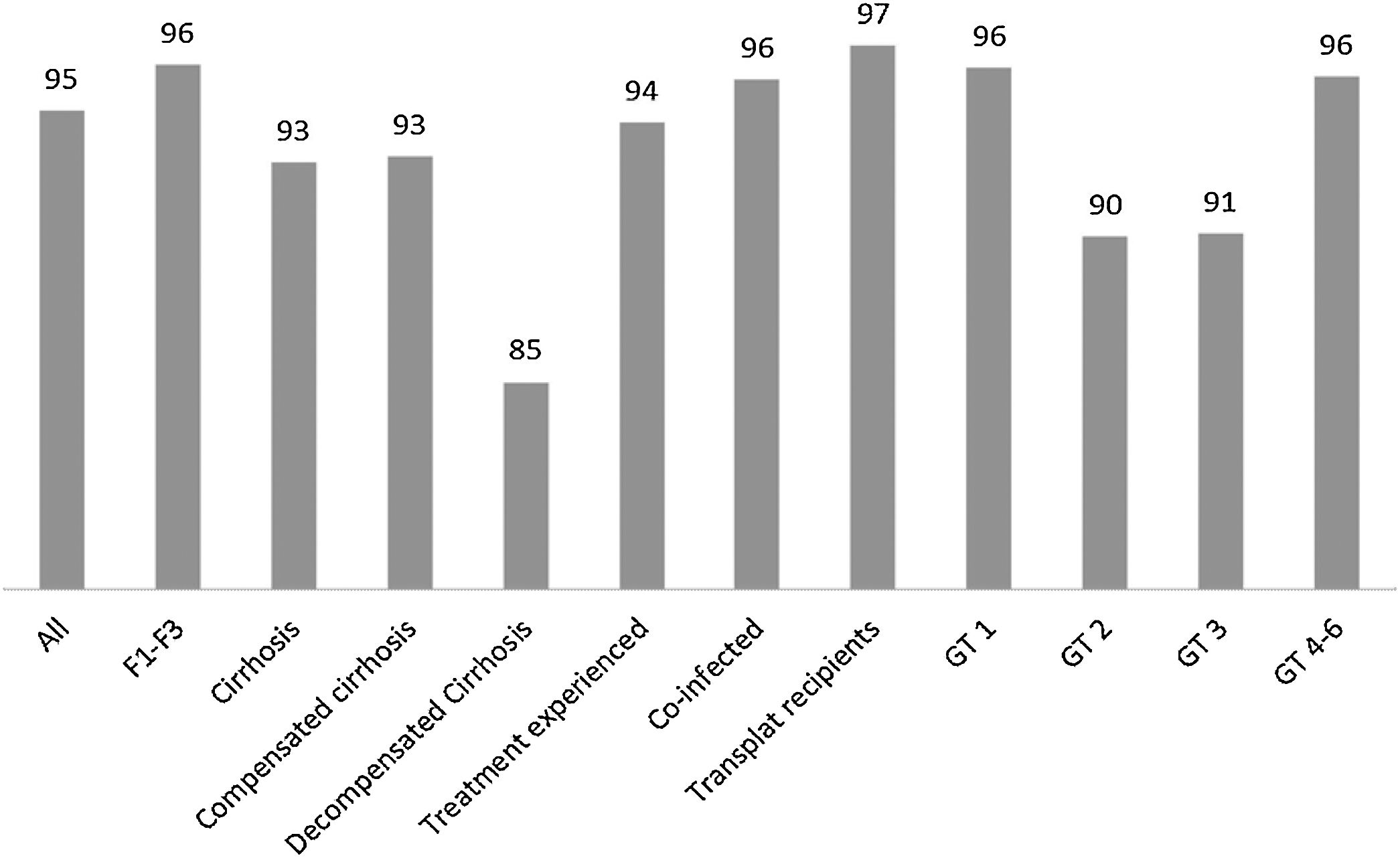
Results3939 patients (60% males, mean age 58±10 years) throughout the country were evaluated. Most had advanced fibrosis or cirrhosis, GT1 and were treated with SOF/DCV or SOF/SIM. Overall SVR rates were higher than 95%. Subjects with decompensated cirrhosis, GT2 and GT3 have lower SVR rates of 85%, 90% and 91%, respectively. Cirrhosis and decompensated cirrhosis in GT1 and male sex and decompensated cirrhosis in GT3 were significantly associated with no SVR. Adverse events (AD) and serious AD occurred in 18% and 5% of those subjects, respectively, but less than 1% of patients required treatment discontinuation.
ConclusionSOF-based DAA regimens are effective and safe in the heterogeneous highly admixed Brazilian population and could remain an option for HCV treatment at least in low-income countries.
According to the World Health Organization (WHO) [1], more than 70,000,000 people are infected with hepatitis C virus (HCV) worldwide. In Latin America, the population frequency of HCV infection was reported to vary from 1.5% to 3% [1–5]. Lower prevalence rates were inasmuch reported in Brazil, where the frequency of HCV was estimated as only 0.7% [6]. However, due to its large population, it is assumed that more than 1.1 million people have acquired HCV infection in Brazil and that over 700,000 of them would have active infection. Hepatitis C is indeed the most common cause of end-stage liver disease (ESLD) requiring liver transplantation in the country [7].
The burden of HCV infection led Brazil to be one of the signatories of the WHO policy of HCV elimination as a major health treat until 2030 [8]. To achieve this goal, the Brazilian Ministry of Health in 2017 shifted its previous policy [6] to prioritize HCV testing mainly to baby-boomers and to grant free access for treatment through the Unified Health System (SUS) with interferon-free direct acting antivirals (DAA) with or without ribavirin (RBV) to all HCV-infected subjects irrespective of disease stage and severity [6]. The first approved regimens in 2015 were sofosbuvir-based therapies±RBV, including sofosbuvir and daclatasvir (SOF/DCV) for genotypes (GT) 1, 3 and 4; sofosbuvir and simeprevir (SOF/SIM) for GT1 and sofosbuvir and ribavirin (SOF/RBV) for GT2 infections [9]. After 2017, new regimens were subsequently approved±RBV, including sofosbuvir and ledipasvir (SOF/LDV), ombistavir, paritaprevir, ritonavir, dasabuvir (OBV/PTVr/DSV) and elbasvir/grazoprevir for GT1 patients±RBV and more recently sofosbuvir/velpatasvir and glecaprevir/pibretasvir [6]. Up to now around 60,000 HCV patients have been treated in Brazil, mainly with SOF-based regimens. Those regimens were shown to induce sustained virological response (SVR) rates over 85–90% in randomized controlled trials (RCT), independently of fibrosis severity or GT [10,11]. Similar results were also obtained in real-world data (RWD) [12] that include large cohorts of patients treated in real-world daily practice settings [13–25]. It is worth to mention, however, that most of those studies were performed in uniform and homogeneous Caucasian populations from Europe and North America [12] and few large-scale RWD have emerged from Latin America [26–28]. The purpose of the present study was to describe the results of a prospective multicenter RWD from Brazil assessing the outcome of HCV treatment with available DAAs from 2015 to 2017.
2Materials and methodsThe Brazilian Society of Hepatology (SBH) created in 2015 after approval of interferon-free regimens for treatment of hepatitis C in Brazil a web-based registry to enroll HCV patients to be submitted to treatment with DAAs in the country. All members of the SBH from every Brazilian region were invited to take part in the study that was financed by SBH. The online database was hosted on the SBH homepage and was designed to include information regarding patient parameters, such as age, gender, self-reported skin color and body mass index (BMI); baseline disease features such as presence of decompensated cirrhosis, organ transplantation, comorbidity, co-infection with hepatitis B or HIV; viral characteristics, such as GT and viral load; type and duration of interferon-free DAA treatment; occurrence of adverse events, severe adverse events leading or not to treatment interruption or modification and SVR at 12 (SVR12) and 24 (SVR24) weeks.
In the database, cirrhosis was classified as compensated or decompensated, according to the occurrence of variceal bleeding, hepatic encephalopathy, ascites, infections or any other liver-related clinical event requiring hospitalization. The presence of cirrhosis was defined by liver biopsy, whenever available, or based on clinical, laboratory, endoscopic and radiological findings. Staging of fibrosis could be evaluated either by liver biopsy or by non-invasive tests such as APRI and FIB-4 and transient elastography (TE). The assessment of SVR was performed through the determination of the viral load at 12 weeks (SVR12) by real-time PCR, with the detection limit of 15IU/ml. The SVR24 was optional in those subjects with SVR12. In addition to adverse effects, information concerning occurrence of liver-related and non-lover-related hospitalizations or death were also included in the database.
The type and duration of DAA treatment was not under physician's discretion and followed the current HCV guidance proposed by the Brazilian Ministry of Health in most patients. During the study period, SOF/RBV and SOF/SIM, SOF/DVC, and OBV/PTVr/DSV were the interferon-free DAA regimens±RBV approved by the Brazilian Regulatory Agency (ANVISA). Due to financial restraints, until 2018 the Brazilian Ministry of Health could cover HCV treatment only to subjects with cirrhosis or advanced fibrosis defined as F3 by the METAVIR classification with the aforementioned SOF-based therapies. Exceptions to those rules included HCV/HIV co-infection, extrahepatic manifestations such as cryoglobulinemia, cutaneous porphyria and severe lichen planus with mucosal involvement and HCV infection after LT and transplantation of other solid organs.
Treatment of HCV patients with lesser degrees of fibrosis or with OBV/PTVr/DSV was only possible in certain subjects with private health insurance. Other DDA regimens, mainly SOF/LDV, could be acquired at that time in Brazil, before regulatory approval, through direct importation of those drugs by HCV patients at their own risk from countries where they were commercially available.
Pretreatment NS5A/NS5B resistance-associated substitutions were not investigated.
The study was approved by the Ethics Committee of participating centers and followed the Ethics guidelines of the Declaration of Helsinki.
Descriptive analysis of demographic data was performed. Continuous variables were described as means±standard deviation and categorical variables as proportions. Continuous variables were compared using the unpaired t-test after testing the premise of normal data distribution. In the univariate analysis, chi-square and Fisher's test were used for categorical variables when appropriate and the odds ratio with 95% confidence intervals was calculated for SVR assessment. p values equal to or less than 0.05 were considered significant. Data is presented in text and tables as mean±standard deviation or percentages. All statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc, Chicago, IL).
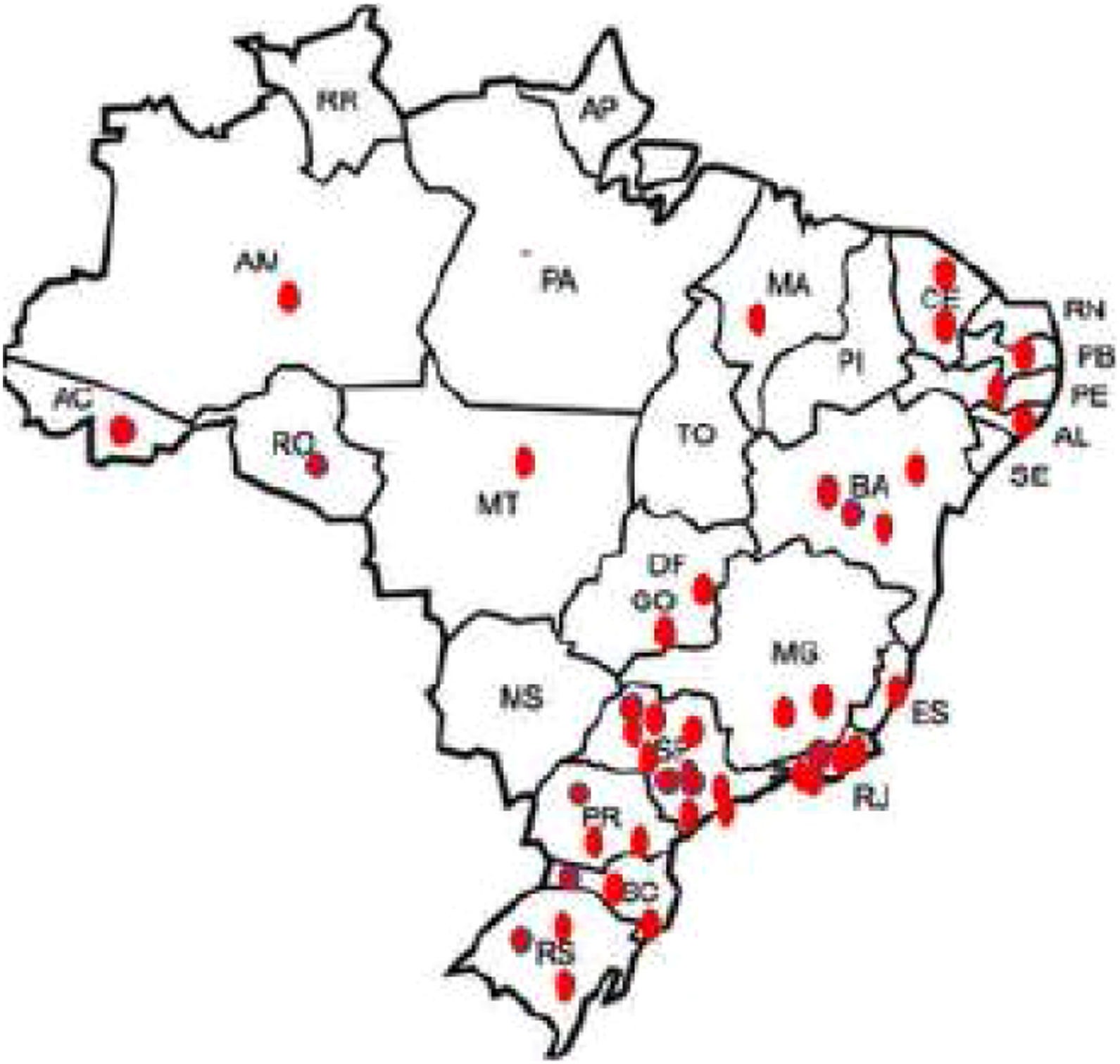
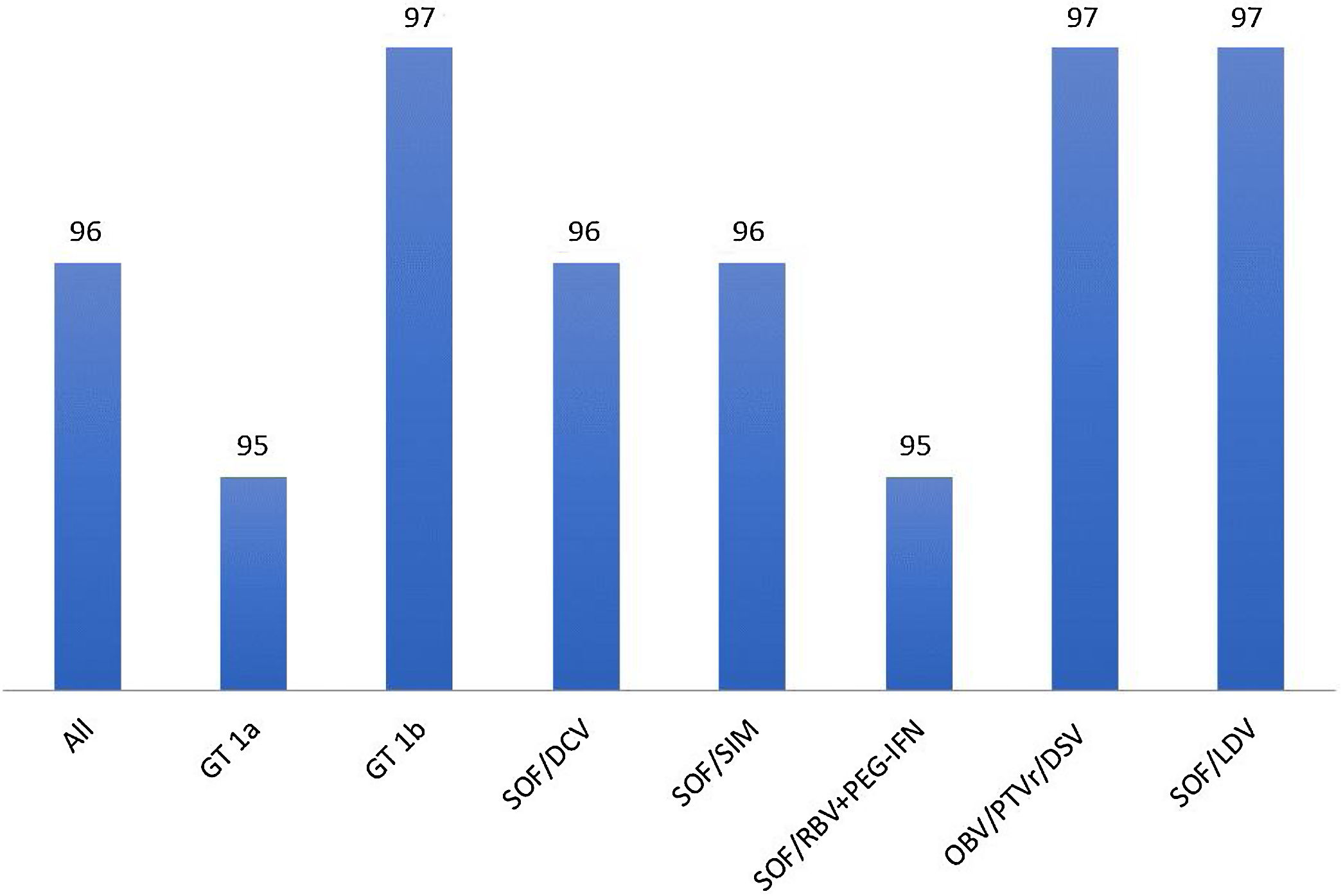
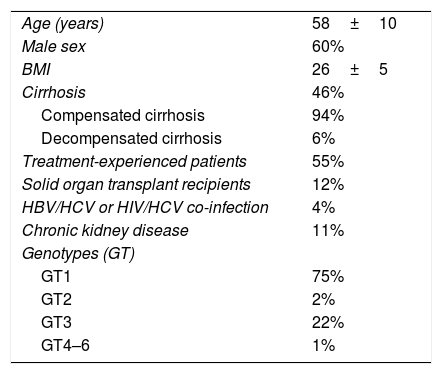
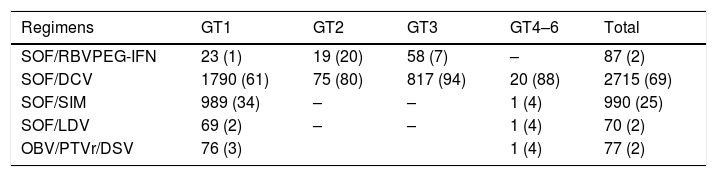
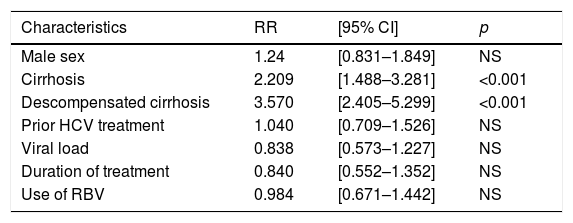
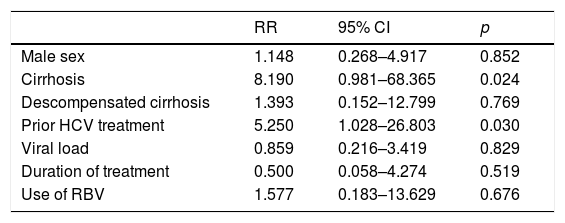
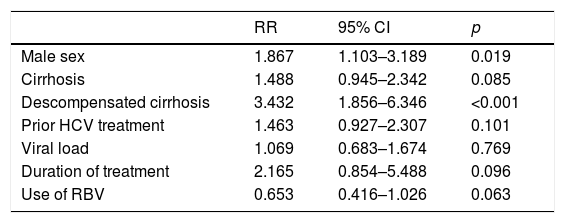
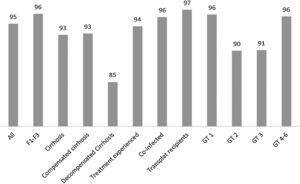
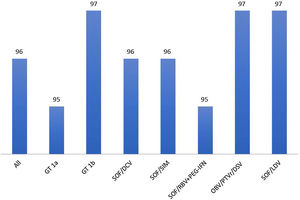
3ResultsFifty-two members of the SBH from different reference centers for HCV treatment from 20 Brazilian states agreed to take part in the study. Participants that included ten or less subjects were excluded from the analysis as per protocol. The distribution of active participating centers is illustrated in Fig. 1. Most of the centers were located in the South and Southeast regions of Brazil, which is in agreement with data from Ministry of Health that show that these regions have the highest density of diagnosed cases of the hepatitis C [6,9]. Overall 4252 patients with hepatitis C were registered in the database. Two hundred eighty-nine and 24 subjects were excluded due to missing data till SVR and informed consent withdrawal, respectively. Clinical and laboratory features of the 3.989 (60% males, mean age 58±10 years) patients included in the study are disclosed in Table 1. Most of them had cirrhosis or advanced fibrosis and were treatment-experienced. The majority of the subjects were GT1 (75%). The treatment regimens employed according to GT are shown in Table 2. Most of the patients with GT1 used either SOF/DCV (61%) or SOF/SIM (34%)±RBV, whereas the majority of GT3 subjects employed SOD/DCV (94%)±RBV. The frequencies of SVR across all GT in different clinical scenarios are illustrated in Fig. 2. SVR rates greater than 90% were achieved in all subjects with the exception of those with decompensated cirrhosis (Fig. 2). From 92 cirrhotic patients with previous carcinoma hepatocellular, 87 of them (94.6%) reached SVR. Concerning HCV genotypes, SVR rates in GT1 HCV patients were greater than 95% with all treatment regimens, with the exception of SOF/RBV±PEG-IFN. Comparing patients with SVR with those without SVR no difference were found for age (58.4±10.8 years×59.1±8.7 years, p=0.527); BMI (26.5±4.4×27.2±4.3kg/m2, p=0.106) and GFR (85.8±22.8×88.4±22.5ml/min, p=0.263). Subjects with GT1a also had slightly lower SVR rates when compared to GT1b (Fig. 3), but by univariate analysis, only the presence of cirrhosis and decompensated cirrhosis were significantly associated with no SVR in GT1 patients (Table 3). Patients with GT2 and GT3 had slightly lower SVR rates when compared to GT1 and GT4–6 (Fig. 2). SVR rates was not statistically different GT2 patients treated either with SOF/DCV (68/76=89.5%) or SOF/RBV±PEG-IFN (18/19=94.7%−X2 0.511, p=0.475). Lower chances of SVR were, nevertheless, observed in patients with cirrhosis or previously experienced subjects (Table 4). No differences in age, BMI and GFR were found between patients with or without SVR. In GT3 patients, SVR rates with SOF/DCV and SOF/RBV±PEG-IFN were 736/817=90.0% and 55/58=94.8%, X2 1.461, p=0.227. The SVR rate was significantly lower in GT3 male sex (513/579=88.6% vs 277/296=93.6% in female sex, X2 5.539, p=0.019) and in patients with decompensated cirrhosis (50/66=75.0% vs 740/809=91.5% in compensated cirrhosis, X2 17,179, p<0.001). Again, in the comparison of patients with and without SVR, no differences were found for age (58.3+9.0×57.7+7.5, t=0.75, p=0.451), BMI (26.2+4.6×27.1+4.5, p=0.140) and GFR (87.1+44.0×90.2+18.4, p=0.237) (Table 5).
Demographic and clinical features of patients with hepatitis C (n=3939).
| Age (years) | 58±10 |
| Male sex | 60% |
| BMI | 26±5 |
| Cirrhosis | 46% |
| Compensated cirrhosis | 94% |
| Decompensated cirrhosis | 6% |
| Treatment-experienced patients | 55% |
| Solid organ transplant recipients | 12% |
| HBV/HCV or HIV/HCV co-infection | 4% |
| Chronic kidney disease | 11% |
| Genotypes (GT) | |
| GT1 | 75% |
| GT2 | 2% |
| GT3 | 22% |
| GT4–6 | 1% |
Chronic kidney disease was defined as estimated glomerular fraction rate less than <60ml/min.
DAA treatment regimens employed in HCV patients with or without RBV according to genotype.
| Regimens | GT1 | GT2 | GT3 | GT4–6 | Total |
|---|---|---|---|---|---|
| SOF/RBVPEG-IFN | 23 (1) | 19 (20) | 58 (7) | – | 87 (2) |
| SOF/DCV | 1790 (61) | 75 (80) | 817 (94) | 20 (88) | 2715 (69) |
| SOF/SIM | 989 (34) | – | – | 1 (4) | 990 (25) |
| SOF/LDV | 69 (2) | – | – | 1 (4) | 70 (2) |
| OBV/PTVr/DSV | 76 (3) | 1 (4) | 77 (2) |
Data in parentheses are percentages.
Factors associated with no SVR in genotype 1 treated patients.
| Characteristics | RR | [95% CI] | p |
|---|---|---|---|
| Male sex | 1.24 | [0.831–1.849] | NS |
| Cirrhosis | 2.209 | [1.488–3.281] | <0.001 |
| Descompensated cirrhosis | 3.570 | [2.405–5.299] | <0.001 |
| Prior HCV treatment | 1.040 | [0.709–1.526] | NS |
| Viral load | 0.838 | [0.573–1.227] | NS |
| Duration of treatment | 0.840 | [0.552–1.352] | NS |
| Use of RBV | 0.984 | [0.671–1.442] | NS |
Factors associated with no SVR in genotype 2 treated patients.
| RR | 95% CI | p | |
|---|---|---|---|
| Male sex | 1.148 | 0.268–4.917 | 0.852 |
| Cirrhosis | 8.190 | 0.981–68.365 | 0.024 |
| Descompensated cirrhosis | 1.393 | 0.152–12.799 | 0.769 |
| Prior HCV treatment | 5.250 | 1.028–26.803 | 0.030 |
| Viral load | 0.859 | 0.216–3.419 | 0.829 |
| Duration of treatment | 0.500 | 0.058–4.274 | 0.519 |
| Use of RBV | 1.577 | 0.183–13.629 | 0.676 |
Predictive factors of no SVR in patients with genotype 3.
| RR | 95% CI | p | |
|---|---|---|---|
| Male sex | 1.867 | 1.103–3.189 | 0.019 |
| Cirrhosis | 1.488 | 0.945–2.342 | 0.085 |
| Descompensated cirrhosis | 3.432 | 1.856–6.346 | <0.001 |
| Prior HCV treatment | 1.463 | 0.927–2.307 | 0.101 |
| Viral load | 1.069 | 0.683–1.674 | 0.769 |
| Duration of treatment | 2.165 | 0.854–5.488 | 0.096 |
| Use of RBV | 0.653 | 0.416–1.026 | 0.063 |
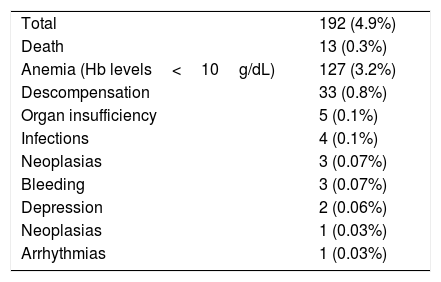
Adverse events occurred in 18% of the patients, mainly asthenia (n=80), headache (n=72) and insomnia (n=45) (Table 6). Overall, less than 1% of the patients had treatment discontinuation. Serious adverse events (SAE), leading or not to modification or discontinuation of treatment, were observed in 4.9% of the cases (Table 5). Overall, less than 1% of the patients had to stop DAA therapy due to SAE. Severe anemia attributed to RBV was the most frequent SAE that resulted in treatment modification, usually reduction in RBV dosages. Thirteen patients died due to decompensation of cirrhosis (n=9) and sepsis (n=4). Comparison of SVR12 with SVR24 results was possible in 1490 patients. All 52 patients without SVR12 remained without SVR24 in the follow-up, whereas only one from 1398 patients who achieved SVR12 turned out to be HCV-RNA positive at week 24.
Frequency of serious adverse events in patients HCV treated with DAA.
| Total | 192 (4.9%) |
| Death | 13 (0.3%) |
| Anemia (Hb levels<10g/dL) | 127 (3.2%) |
| Descompensation | 33 (0.8%) |
| Organ insufficiency | 5 (0.1%) |
| Infections | 4 (0.1%) |
| Neoplasias | 3 (0.07%) |
| Bleeding | 3 (0.07%) |
| Depression | 2 (0.06%) |
| Neoplasias | 1 (0.03%) |
| Arrhythmias | 1 (0.03%) |
The present study was a prospective multicenter real-life study from Brazil assessing the outcome of HCV treatment with available DAAs from 2015 to 2017. Real-world data concerning HCV treatment are very important to verify its effectiveness and safety in daily practice outside the scope of highly controlled RCTs [12]. Few RWD concerning safety and efficacy of DAA regimens for treatment of HCV virus were reported in Latin America, including Brazil [26–28]. This could be an important issue regarding HCV treatment outcomes, particularly in Brazil whose population is of highly admixed origin and have marked regional disparities in demographic and socioeconomic indices and access to healthcare facilities that could influence adherence to treatment and retention in care, as pointed out elsewhere [7]. Most patients were submitted to treatment in public healthcare centers. According to Brazilian Ministry of Health policy in 2015, due to financial constraints, to be treated all patients should have either cirrhosis or advanced fibrosis [9]. Due to these reasons, our RWD for HCV treatment were derived from a large-cohort of patients of highly admixed origin from all parts of the country. As stated, most of them had GT1 and advanced fibrosis or cirrhosis and were submitted to treatment with SOF-based regimens.
Overall SVR rates higher than 95% were observed across all genotypes with slightly lower SVR observed in subjects with decompensated cirrhosis and GT2 and G3 HCV infection. With the exception of SVR in GT2 HCV patients, our results were in accordance to previously published real-world data [10,12]. The lower than expected SVR rates in GT2 were probably due to the use of the less effective SOF/RBV regimen in 20% of those patients. Response rates over 95% were observed in all GT1 HCV+patients, with the exception of those subjects with GT1a subtype and those treated with SOF/RBV+PEG-IFN who had slightly lower SVR rates (still higher than 90%). Different from previous real-life reports [14,29,30], SOF/SIM regimen in the present study was not shown to be inferior to SOF/DCV in respect to SVR rate. Absence of SVR was associated with cirrhosis and decompensated cirrhosis in GT1, cirrhosis and prior HCV treatment in GT2 and male sex and decompensated cirrhosis in GT3 HCV+patients. The influence of decompensated cirrhosis in SVR rates was extensively reported in RCT and real life studies particularly in GT1 and GT3 [13–25]. The impact of cirrhosis and prior HCV treatment in GT2 HCV+patients was probably due to the employment of less efficient DAA regimens that were available at that time for treatment of GT2. On the contrary, as expected, most of the patients (64%) without SVR were GT3 with cirrhosis (50%) in the present study (data not shown). This study has some limitations. Investigation of resistant-associated substitutions (RAS) was not performed and it is well known that some of them, specifically those related to NS5A region, are associated with lower response to therapy [31,32]. However, some studies have demonstrated that the frequency of clinically significant baseline RAS in Brazilian population is not relevant [33,34] and probably has not affected the SVR results. In accordance with these data, a recent Brazilian study has demonstrated that despite the occurrence of baseline RAS in a sample of 132 HCV-infected patients, the response rate to SOF/DCV treatment was very high, of 95.4% [35].
Another limitation was the lack of systematic investigation of hepatocellular carcinoma. Patients with hepatitis C and liver cancer may be less likely to achieve cure than those without liver cancer. However, in this study, the group of patients with past history of hepatocellular carcinoma had a satisfactory SVR.
In summary, this RWD from Brazil is the largest case-series reported from Latin America using second generation DAAs showing overall SVR rates higher than 95% in patients of highly admixed origin and different socioeconomic status from different parts of the country. Those SVR rates were achieved even with SOF/SIM, a drug regimen that is no more recommended in most of international guidelines for HCV treatment, (including ours) due to suboptimal SVR rates when compared to other regimens [14,29,30]. SVR rates with SOF/DCV were also higher than 90% across GT. This could reinforce the WHO policy of still recommend SOF/DCV as a cost-effective pangenotypic drug regimen, despite the advent of newer DAA regimens.
5ConclusionIn conclusion, those results support the effectiveness and safety of SOF-based regimens in the heterogeneous Brazilian population and still suggest a role of those drug regimens in the treatment of HCV at least in low-income countries, where cost-effectiveness is an important issue to achieve WHO recommendation for HCV.AbbreviationsAD adverse events Brazilian Regulatory Agency Brazilian Society of Hepatology daclatasvir direct antiviral agents genotype simeprevir sofosbuvir Unified Health System sustained virological response
There is no conflict of interest in this article.
Mrs. Ayk Helena Barbosa Martins for the study coordenation.