A limited number of medications are typically considered for the management of hepatic encephalopathy occurring as a complication of transjugular intrahepatic portosystemic shunt (TIPS) placement. Multiple alternative compounds aimed at disrupting ammoniagenesis are or will soon be available, though their use tends to be limited by a lack of large data sets and of clinical awareness. In this review, we provide a targeted overview of the mechanisms and availability of five anti-ammoniagenic compounds (sodium phenylbutyrate, glycerol phenylbutyrate, sodium benzoate, L-ornithine L-aspartate, and ornithine phenylacetate) identified as possibly useful alternative therapeutic agents for cirrhotic encephalopathy. Three of these medications have been FDA approved for use in congenital urea cycle disorders only, while two are under active investigation for use in cirrhotic patients. In spite of limitations posed by cost and comorbidities, familiarity with these options may prove beneficial in cases refractory to conventional management.
Transjugular intrahepatic portosystemic shunt (TIPS) placement is typically pursued in order to control complications of portal hypertension like ascites accumulation or variceal bleeding that have become refractory to medical or endoscopic management. The procedure, typically performed under radiologic guidance, involves introduction of a specialized catheter though one of the jugular veins into the hepatic venous system. A needle is then used to pierce the liver parenchyma, forming a tract with the portal venous system that is subsequently balloon-dilated and bolstered with an expandable stent.1 By providing an auxiliary pathway for blood flow from the splanchnic circulation into the systemic circulation, the shunt thus attenuates portal venous pressure and its associated consequences.
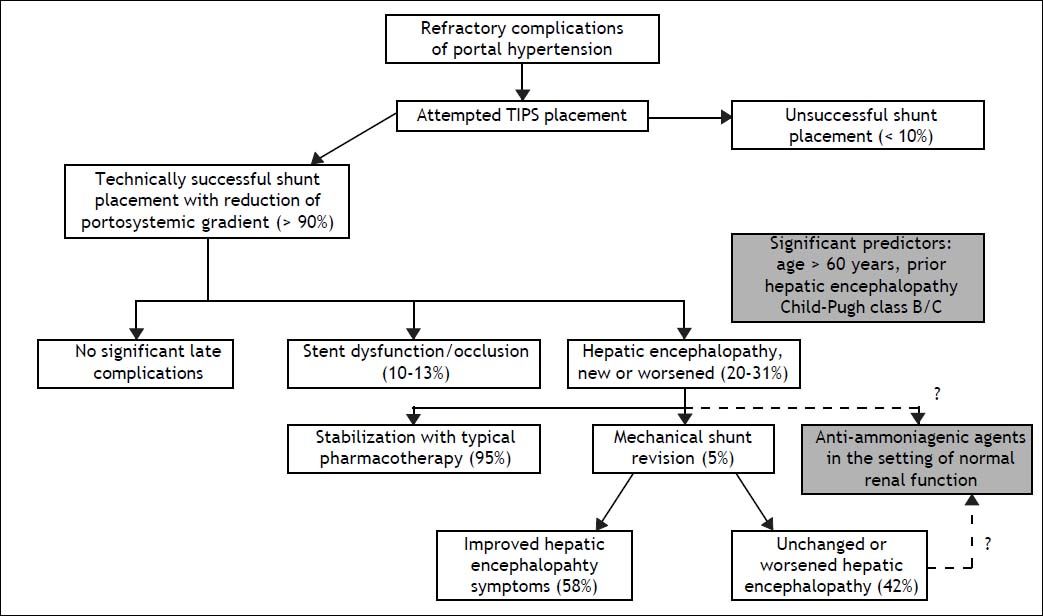
Immediate technical success rates for shunt placement are typically greater than 90%. One major adverse consequence, however, is new or worsened hepatic encephalopathy (HE), which complicates roughly 20-31% of all TIPS procedures (Figure 1).2 HE encompasses a wide spectrum of cognitive impairment ranging from mild distractibility and forgetfulness to frank coma.3 Onset of post-TIPS encephalopathy typically occurs within a few months following the procedure. Symptoms may improve with time, due to either progressive shunt stenosis or the brain’s adaptation to an increased neurotoxin load, though especially with the use of newer stents that remain patent for longer periods of time, encephalopathy may develop more than a year after the procedure.4 According to a recent systematic review, the most significant risk factors for the development of post-TIPS encephalopathy include advanced age, the presence of encephalopathy symptoms prior to shunt placement, and significantly diminished liver function as indicated by a high Child-Pugh score.5
Important distinctions bear mentioning within the larger category of encephalopathy due to portal hypertension or portal-systemic shunts. The first major division is between minimal and overt hepatic encephalopathy, the former describing a subclinical entity that is diagnosed by neuropsychometric testing but nonetheless leads to a reduction in quality of life.6,7 Overt HE is diagnosed on the basis of clinical symptoms and is sub-divided into persistent and episodic encephalopathy, either of which can follow TIPS placement. Episodic HE may be spontaneous or precipitated, and resolution of an underlying encephalopathy trigger (infection, gastrointestinal bleeding, or electrolyte disturbances, for example) is often effective at improving symptoms. Persistent HE may be variable in severity, though as the terminology suggests, symptoms never fully remit and may become dependent on continued therapy.3
Treatment for post-TIPS encephalopathy, as with HE of other etiologies, most commonly involves administration of non-absorbable disaccharides, other cathartics, or antibiotics. Among the estimated 5% of patients in whom these agents fail, shunt downsizing, shunt occlusion, or liver transplantation may be needed to mitigate symptoms, though these methods are not always useful or viable. One large recent analysis of TIPS revisions showed that hepatic encephalopathy symptoms improved in 58% of cases, with associated risks including recurrent bleeding, recurrent ascites, mesenteric infarction, and major infection.8 Common agents like lactitol and rifaximin have been evaluated in a prophylactic context after TIPS, though neither agent was shown to be effective in reducing the incidence of post-procedural encephalopathy.9 Novel stepwise approaches to medication administration and stent size adjustment have been proposed, but these approaches remain unstudied.10
Medication pricing has emerged as another important mediator of the usual approach to HE therapy. A 2007 decision analysis illustrated the fact that although rifaximin is equivalent or superior to lactulose at improving encephalopathy symptoms in head-to-head trials, the relative inexpensiveness of the latter agent has made it more cost-effective as monotherapy.11,12 To our knowledge, such a comparison study or cost-effectiveness analysis has not been performed with specific attention to post-TIPS encephalopathy. Considering TIPS itself in economic terms, one prior analysis estimated initial procedural costs in the range of $21,000, with final costs over five years of follow-up exceeding $70,000.13 However these numbers may vary in individual cases, the associated utility is largely negated when a shunt must be reversed in the event of refractory encephalopathy.
PathogenesisExperimental evidence has given rise to multiple putative mechanisms for the pathogenesis of hepatic encephalopathy, including among others the impaired clearance of various neurotoxic compounds, increased neuroinhibitory tone secondary to type A gamma-aminobutyric acid (GABAA) signaling,14 and cerebral edema generated by hyponatremia and inflammatory mediators.15 Many of these hypotheses describe interactive and mutually dependent processes within a biochemically complex disorder, but among them, the accumulation of neurotoxins, specifically ammonia, remains central.
Likewise for post-TIPS encephalopathy, the most widely held pathogenetic model involves an increased shunting of ammonia from the portal veins into the systemic circulation via the new conduit created across the liver.16 This hypothesis is supported by the observed risk of encephalopathy among patients with portal-systemic shunts existing in the absence of liver dysfunction.17 This risk is certainly compounded by the presence of cirrhosis, however, suggesting that the TIPS procedure may further diminish an already limited hepatic reserve.18 Research in rat models has also suggested that the intestinal activity of glutaminase, an enzyme directly involved in ammoniagenesis, is increased following portocaval shunt formation.19
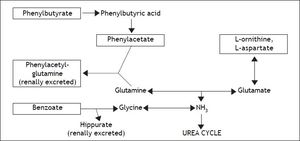
Several methods of HE prevention and therapy have been trialed, exploiting a wide range of potential mechanisms. Dietary restriction and avoidance of neuroactive medications, for example, are frequently utilized as adjunctive strategies to reduce the risk of encephalopathy development. This review will dwell primarily on alternative therapeutic agents specifically targeting the disruption of ammonia metabolism (Figure 2) toward the goal of treating overt encephalopathy following TIPS placement. Indeed, the most commonly utilized medical therapies at present for post-TIPS encephalopathy (and HE in general) are already directed toward decreasing ammonia production and increasing its clearance. Non-absorbable disaccharides like lactulose or lactitol are typical initial agents, whose metabolites acidify the gut, inhibit ammoniagenic enzymes (urease), and favor ammonia’s conversion to ammonium, which does not enter the bloodstream. Antibiotics such as the non-absorbable rifaximin and neomycin or the partially absorbed metronidazole are typically utilized next and function by decontaminating the gut of urease-producing bacteria.20
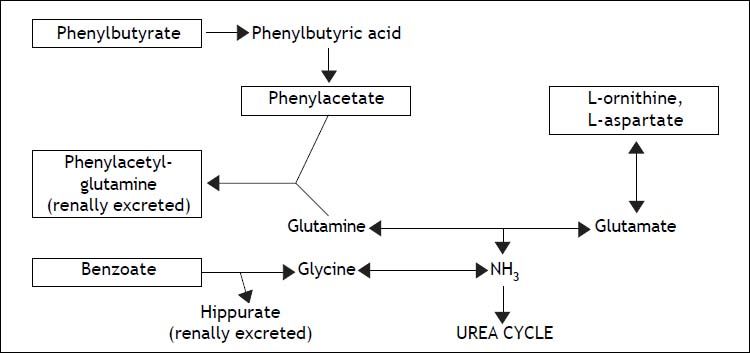
The steps of ammoniagenesis targeted by various third-line encephalopathy therapies (listed in boxed text). Phenylbutyrate, phenylacetate, and benzoate lead to the formation of renally excreted molecules (listed in bold), while ornithine and aspartate increase glutamate production, leading in turn to the preferential synthesis of glutamine.
Newer agents (Table 1) conceived as competitive intermediates within ammonia metabolism pathways have a less robust basis of evidence as HE therapy, though research is ongoing. Phenylbutyrate is one such compound. Its bioactive derivative, phenylacetate, complexes with glutamine to form the renally excreted molecule phenylacetylglutamine, a process that reduces the amount of nitrogenous substrate available for ammoniagenesis.21 Sodium phenylbutyrate (BuphenylTM, Ucyclyd Pharma, Scottsdale, AZ, USA) has been FDA approved particularly for use in chronic hyperammonemic states associated with urea cycle disorders.22 No formal trials have been initiated on its use in cirrhotic HE, much less with particular application to post-TIPS encephalopathy; anecdotal evidence at our institution, however, has been promising in the acute setting (despite acute hyperammonemia being a labeled contraindication to the use of this agent). Potential obstacles to the long-term use of sodium phenylbutyrate in cirrhotic patients include poor palatability and excessive sodium load, the latter potentially predisposing to ascites accumulation (1 gram of the drug delivers approximately 125 milligrams of sodium, and typical adult doses of sodium phenylbutyrate range from 10-20 g/day).23
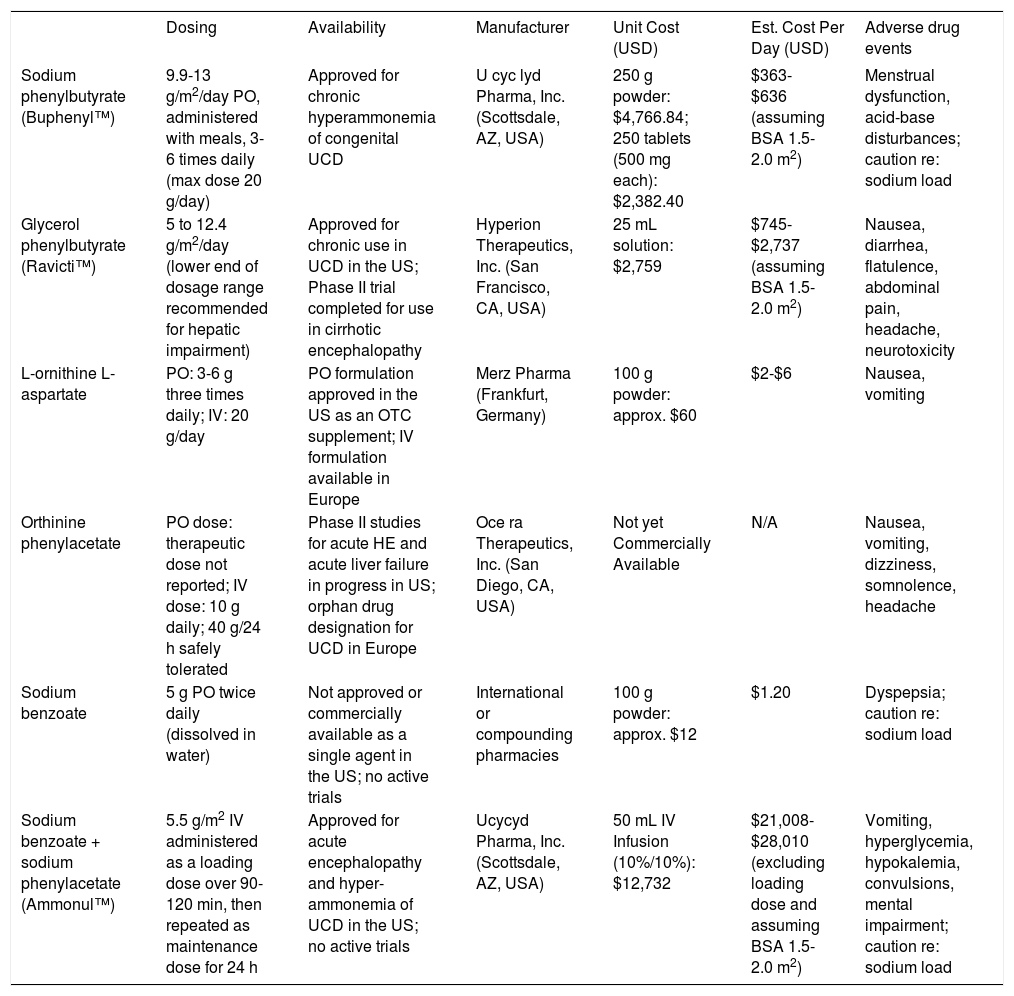
Anti-ammoniagenic agents of potential utility in post-TIPS encephalopathy. Pricing information was obtained by telephone from a local compounding pharmacy in the US (for sodium benzoate) and pharmaceutical references and/or commercial pharmacies (for other agents). Pricing information is thought to be accurate as of August 2013; some variation is to be expected based on the distributor.
| Dosing | Availability | Manufacturer | Unit Cost (USD) | Est. Cost Per Day (USD) | Adverse drug events | |
|---|---|---|---|---|---|---|
| Sodium phenylbutyrate (Buphenyl™) | 9.9-13 g/m2/day PO, administered with meals, 3-6 times daily (max dose 20 g/day) | Approved for chronic hyperammonemia of congenital UCD | U cyc lyd Pharma, Inc. (Scottsdale, AZ, USA) | 250 g powder: $4,766.84; 250 tablets (500 mg each): $2,382.40 | $363-$636 (assuming BSA 1.5-2.0 m2) | Menstrual dysfunction, acid-base disturbances; caution re: sodium load |
| Glycerol phenylbutyrate (Ravicti™) | 5 to 12.4 g/m2/day (lower end of dosage range recommended for hepatic impairment) | Approved for chronic use in UCD in the US; Phase II trial completed for use in cirrhotic encephalopathy | Hyperion Therapeutics, Inc. (San Francisco, CA, USA) | 25 mL solution: $2,759 | $745-$2,737 (assuming BSA 1.5-2.0 m2) | Nausea, diarrhea, flatulence, abdominal pain, headache, neurotoxicity |
| L-ornithine L-aspartate | PO: 3-6 g three times daily; IV: 20 g/day | PO formulation approved in the US as an OTC supplement; IV formulation available in Europe | Merz Pharma (Frankfurt, Germany) | 100 g powder: approx. $60 | $2-$6 | Nausea, vomiting |
| Orthinine phenylacetate | PO dose: therapeutic dose not reported; IV dose: 10 g daily; 40 g/24 h safely tolerated | Phase II studies for acute HE and acute liver failure in progress in US; orphan drug designation for UCD in Europe | Oce ra Therapeutics, Inc. (San Diego, CA, USA) | Not yet Commercially Available | Ν/A | Nausea, vomiting, dizziness, somnolence, headache |
| Sodium benzoate | 5 g PO twice daily (dissolved in water) | Not approved or commercially available as a single agent in the US; no active trials | International or compounding pharmacies | 100 g powder: approx. $12 | $1.20 | Dyspepsia; caution re: sodium load |
| Sodium benzoate + sodium phenylacetate (Ammonul™) | 5.5 g/m2 IV administered as a loading dose over 90-120 min, then repeated as maintenance dose for 24 h | Approved for acute encephalopathy and hyper-ammonemia of UCD in the US; no active trials | Ucycyd Pharma, Inc. (Scottsdale, AZ, USA) | 50 mL IV Infusion (10%/10%): $12,732 | $21,008-$28,010 (excluding loading dose and assuming BSA 1.5-2.0 m2) | Vomiting, hyperglycemia, hypokalemia, convulsions, mental impairment; caution re: sodium load |
UCD: urea cycle disorders. OTC: over-the-counter.
An alternative formulation, glycerol phenylbutyrate (GPB) (RavictiTM, Hyperion Therapeutics, San Francisco, CA, USA), bypasses these obstacles and is under active investigation for HE prevention among cirrhotic patients, having already been observed to be safe and well tolerated in this patient population.24 GPB has recently received FDA approval, but like sodium phenylbutyrate, its labeled use is currently limited to urea cycle disorders.25 The agent is currently available as an oral liquid, with a daily cost slightly higher than that of sodium phenylbutyrate. Preliminary results of a randomized double-blind Phase 2 trial of GPB among 178 cirrhotic patients with a history of encephalopathy suggest that, in combination with a standing regimen of lactulose and/or rifaximin, GPB is superior to placebo for preventing new HE events and reducing the length and frequency of future HE hospitalizations.26
L-ORNITHINE-L-ASPARTATE AND ORNITHINE PHENYLACETATEOther compounds aimed at disrupting ammonia synthesis in hepatic encephalopathy include L-ornithine-L-asparate (LOLA) (Hepa-Merz®, Merz Pharma, Frankfurt, Germany) and ornithine phenylacetate (Ocera Therapeutics, San Diego, CA, USA). Ornithine is metabolized in vivo to glutamate, which in turn is thought to facilitate the diversion of nitrogen in excess ammonia toward glutamine synthesis and excretion.27 Results have been generally favorable regarding the efficacy of LOLA in treating HE. A recent meta-analysis identified three high-quality randomized controlled trials of LOLA among a total of 212 patients with chronic Stage I and II HE;28 the largest of the included trials, with 126 patients, evaluated the effects of intravenous dosing,29 while the others studied the drug’s oral formulation. All concluded that LOLA improved symptoms of clinically overt encephalopathy relative to either lactulose or placebo.
Among patients with HE related to acute liver failure, however, a randomized trial of 201 subjects showed no reduction in mortality or encephalopathy grade with intravenous LOLA as compared with placebo.30 Furthermore, intervention periods in trials of LOLA have often been short (four to seven days in the studies mentioned above), and some investigators have suggested that the agent’s clinical effects are transient with risk of rebound hyperammonemia on discontinuation (an observation specifically reported from a cohort of eight cirrhotic patients, though without associated details of their baseline characteristics.)31 LOLA has also been studied with particular attention to the post-TIPS setting, though results are limited in their interpretability. A crossover trial of fifteen cirrhotic patients, seven with TIPS and eight without, challenged participants with a glutamine load (promoting ammoniagenesis) and treated them with either intravenous LOLA or placebo; non-TIPS subjects demonstrated an improvement in choice response time with LOLA relative to placebo, but no reduction in psychometric testing performance could be generated in either treatment arm among subjects with TIPS.32
In addition to providing ornithine as a driver of glutamine synthesis, ornithine phenylacetate (OP) complexes with glutamine to form a molecule that can be renally excreted (by a mechanism similar to that of phenylbutyrate, a metabolic precursor of phenylacetate, as mentioned above), an action hypothesized as a potential basis for more durable symptomatic benefit.22 Much of the published evidence at present is derived from animal models of cirrhosis and liver failure, where significant reductions have been noted in serum ammonia concentrations, brain edema, and neurophysiologic deficits as measured by motor-evoked potentials.33,34 A small observational study of ten human cirrhotic patients with recent gastrointestinal bleeding demonstrated the relative safety of OP administration in this population along with reliable reduction in serum ammonia concentration and correlative increase in urinary phenylacetylglutamine, the excreted metabolite.35 Large clinical trials of OP have yet to be published, though it has received orphan drug and fast track designations from the FDA, and Phase II studies are in progress investigating the agent’s utility in cirrhotic hepatic encephalopathy and acute liver failure respectively.36,37
Sodium BenzoateLimited evidence also supports the utility of sodium benzoate in diverting nitrogen from ammonia synthesis pathways, specifically by complexing with glycine to form the renally excreted molecule hippurate.38 Like phenylbutyrate, benzoate was initially used in urea cycle disorders, but data from one randomized controlled trial of 74 patients with HE (in the setting of underlying cirrhosis, except for 4 cases with portosystemic shunt) also demonstrated efficacy in the treatment of cirrhotic encephalopathy, with benefits equivalent to those seen with lactulose administration (in combination with ancillary standard of care measures, including tap water enemas and dietary protein restriction.) In that study, sodium benzoate therapy (5 grams twice daily) was noted to be thirty times cheaper than the disaccharide alternative at the time and location of publication.39
Conflicting data have been reported, however, on the utility of sodium benzoate in reducing serum ammonia levels outside the setting of overt encephalopathy. A subsequent trial of six cirrhotic patients demonstrated relative hyperammonemia following sodium benzoate administration, both at baseline and after a glumatine challenge.40 The authors suggest that the post-treatment effects seen in the larger randomized trial may have been attributable to the standard of care provided in both arms rather than the experimental agent in question. While emerging from a much smaller sample size, these results resonate with doubt raised by earlier animal studies regarding the direction of effect of sodium benzoate on serum ammonia levels.41
As with sodium phenylbutyrate, caution would seem advisable in a cirrhotic population regarding the high sodium load associated with long-term administration of this medication. Per molecular-weight calculations, assuming administration of an additivefree powder, approximately 160 milligrams of sodium would be delivered per gram of sodium benzoate, corresponding with 1.6 g of sodium per day of therapy at usual doses. One observational study of sodium benzoate administered daily over six months to eighteen patients with chronic cirrhotic encephalopathy notably demonstrated no significant fluid retention at monthly follow-up, though the severity of participants’ liver disease is difficult to ascertain.42 From our anecdotal experience, the palatability of this dissolved compound is also poor and therefore potentially limiting.
Oral sodium benzoate has not been FDA approved for medicinal use, nor is it commercially manufactured as a single agent in North America, though it may be obtained from compounding pharmacies at minimal expense. Sodium benzoate is also available as an intravenous combination therapy with sodium phenylacetate (AmmonulTM, Ucyclyd Pharma, Scottsdale, AZ, USA), labeled for treatment of acute hyperammonemia in the setting of urea cycle disorders.43 This formulation is markedly more expensive than the other agents discussed herein, particularly for the higher dosages that would be required in adult patients; studies of its use in urea cycle disorders suggest that it is typically employed as a short-term bridge to the successful administration of oral therapy.44 Published evaluations of this combination therapy in an adult cirrhotic population are rare; one small crossover trial of oral administration among eight patients with portosystemic encephalopathy demonstrated improvement in encephalopathy grade in the majority of participants with dual therapy, greater than the improvement seen with either agent alone.45
ConclusionPost-procedure encephalopathy poses a significant challenge to the long-term management of cirrhotic patients requiring TIPS. Shunt downsizing and reversal typically follow trials of non-absorbable disaccharides and antibiotics, opposing the initial intervention’s intended benefit and introducing further risk of procedural morbidity. This review seeks to promote familiarity with a richer field of pharmacologic options by which encephalopathy might be reversed and an otherwise functional shunt salvaged.
Several caveats bear mentioning prior to the clinical implementation of these medications. The generalizability of research into third-line agents for acute or chronic HE management is often limited by the grade and mechanism of encephalopathy studied, as well as the potential concurrent use of other HE medications. Few clinical trials have been performed with particular attention to post-TIPS encephalopathy. Moreover, newer compounds have typically not been specifically studied in the setting of other chronic impairments, such as renal dysfunction, an important point of caution given the reliance of many of the aforementioned nitrogen-wasting agents on renal clearance for their mechanism of action.
Cost is another significant consideration when evaluating the feasibility of these third-line options. As mentioned above, the high price of intravenous combination therapy with sodium benzoate and sodium phenylacetate makes it unlikely to be useful as an agent for cirrhotic patients with chronic encephalopathy symptoms. Oral sodium benzoate and LOLA are comparatively inexpensive but respectively limited by concerns regarding excessive sodium administration, exacerbation of hyperammonemia, and transient symptomatic benefit. Specific price points have yet to be set for those agents still under investigation, but in broad terms, the cost-benefit analysis of initiating one of these less conventional medications in a cirrhotic patient will likely hinge significantly on its perceived utility as an acute versus chronic management strategy. Our anecdotal experience suggests that at least one of these agents (sodium phenylbutyrate) can provide sustained symptomatic benefit and reduced need for recurrent hospitalization within the context of a limited course of therapy following TIPS placement. Further investigation is still pending with respect to the wider applicability of anti-ammoniagenic agents in cirrhotic and post-procedure encephalopathy.
Abbreviations- •
GPB: glycerol phenylbutyrate.
- •
HE: hepatic encephalopathy.
- •
LOLA: L-ornithine L-aspartate.
- •
OP: ornithine phenylacetate.
- •
TIPS: transjugular intrahepatic portosystemic shunt.