Recent innovations in the field of liver transplantation have led to a wealth of new treatment regimes, with potential impact on the onset of de novo malignancies (DNM). The aim of this multicenter cohort study was to provide contemporary figures for the cumulative incidences of solid and hematological DNM after liver transplantation.
MethodsWe designed a retrospective cohort study including patients undergoing LT between 2000 and 2015 in three Italian transplant centers. Cumulative incidence was calculated by Kaplan–Meyer analysis.
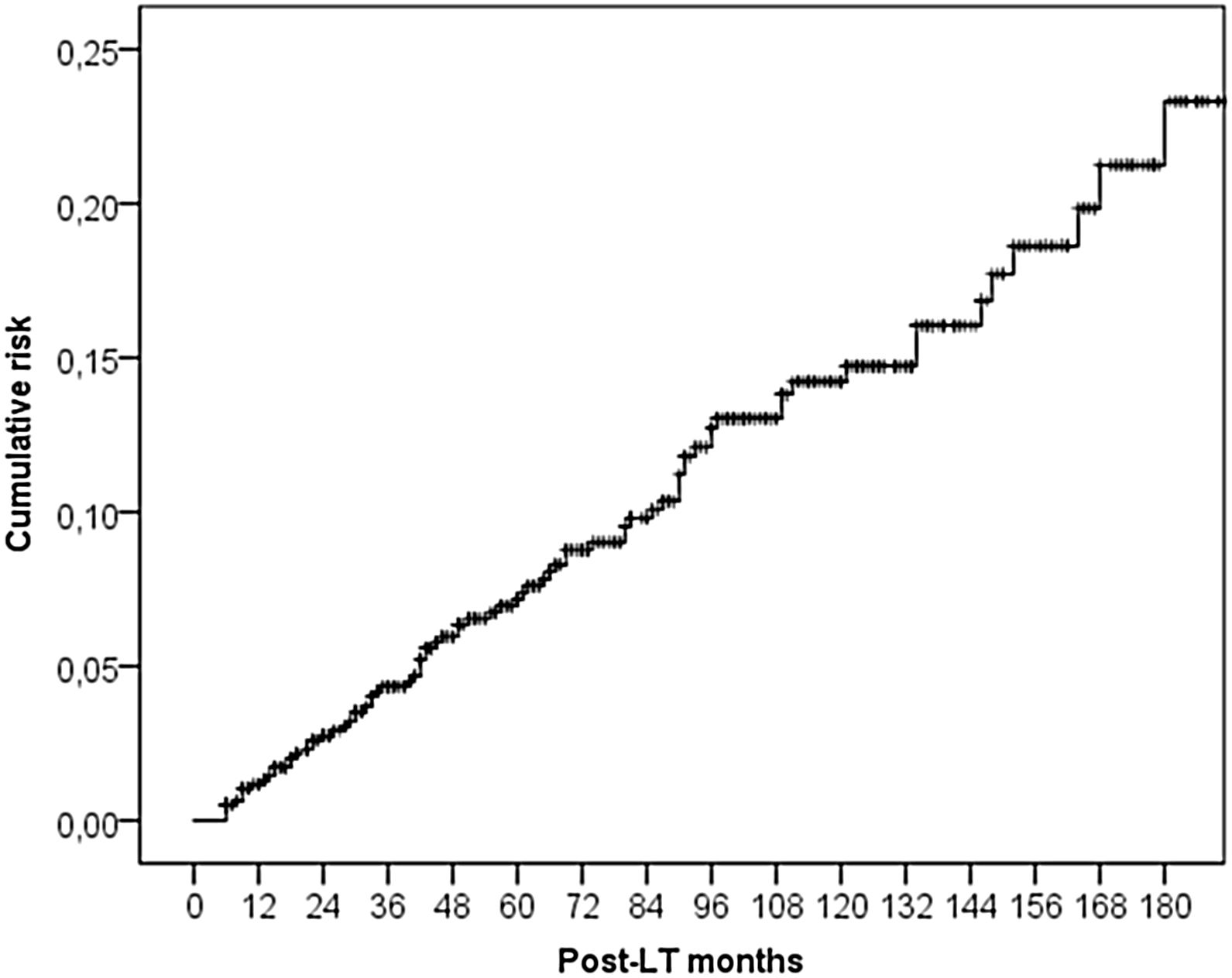
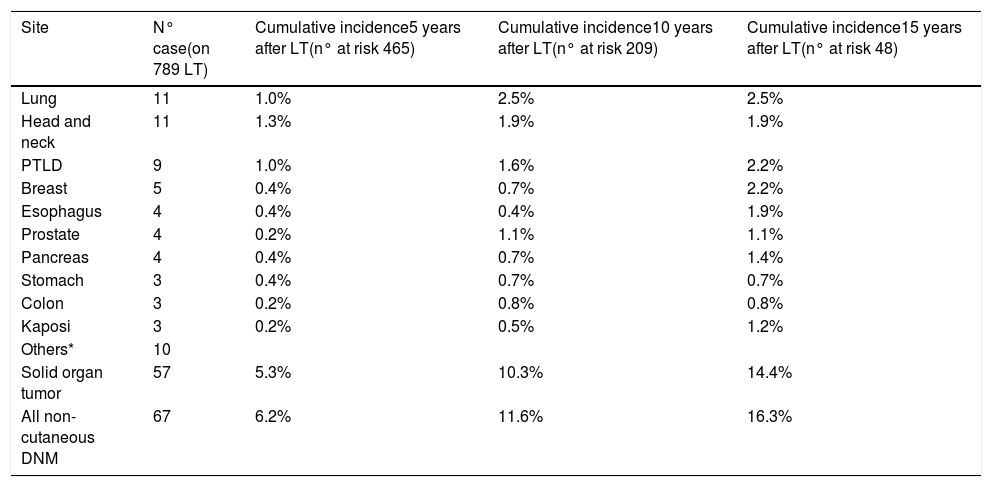
ResultsThe study included 789 LT patients with a median follow-up of 81 months (IQR: 38–124). The cumulative incidence of non-cutaneous DNM was 6.2% at 5-years, 11.6% at 10-years and 16.3% at 15-years. Post-Transplant Lymphoproliferative Disorders (PTLD) were demonstrated to have a cumulative incidence of 1.0% at 5-years, 1.6% at 10-years and 2.2% at 15-years. Solid Organ Tumors (SOT) demonstrated higher cumulative incidences – 5.3% at 5-years, 10.3% at 10-years and 14.4% at 15-years. The most frequently observed classifications of SOT were lung (rate 1.0% at 5-years, 2.5% at 10-years) and head & neck tumors (rate 1.3% at 5-years, 1.9% at 10-years).
ConclusionsLung tumors and head & neck tumors are the most frequently observed SOT after LT.
Long term graft survival after liver transplantation (LT) currently exceeds 55% at 10 years [1] but 10-year survival is 21% lower than expected for the general population [2]. Malignancies (both recurrent and de novo) are the most common cause of death in patients surviving at least one year after LT [2,3] and the cumulative risk increases with age and the temporal distance from the transplant [4]. In an analysis of the Scientific Registry of Transplant Recipients from 1987 to 2009, LT patients were found to carry an 11-fold higher risk of developing non-skin de novo malignancy (DNM) than the general population [5].
The most important precipitant of DNM in transplanted patients is chronic immunosuppression [6]. Immunosuppressive therapy increases the risk of neoplasia through several mechanisms including reduced immunological surveillance, increased susceptibility to infections with possible oncogenic effects, induction of insulin resistance and a speculative direct mutagenic action [7,8]. Furthermore, the effects of conventional risk factors for neoplasia (i.e., alcohol, smoking, pre-cancerous lesions) can be exaccerbated by decreased immuno-competence [9].
Recent innovations in the field of LT have led to changes in the management of transplant patients, with a potential impact on the onset of DNM after LT. Calcineurin inhibitors (cyclosporin and tacrolimus) have been consistently associated, in a dose-dependent fashion, with an increased risk of both Hepatocellular Carcinoma (HCC) recurrence and DNM incidence [10], hence minimizing levels of immunosuppression is widely practiced. The combination of antiproliferative and immunosuppressant activity of mTOR inhibitors (everolimus and sirolimus) make them attractive in post-LT setting. Despite the promising biological rationale, retrospective studies and an open-label clinical trial did not convincingly demonstrate lower rates of HCC recurrence in patients receiving mTOR inhibitors [10,11]. Despite early evidence of lower incidence of DNM in patients treated with mTOR inhibitors these benefits remain largely unsubstantiated [12].
Regarding indications, in recent years LT performed for non-alcoholic steatohepatitis (NASH) increased significantly whereas transplant for Hepatitis C virus (HCV) significantly reduced as a consequence of the introduction of the new direct acting antivirals [13]. Since NASH may be a risk factor for extra-hepatic cancers, such colorectal cancer [14], this may have an impact on DNM incidence in future.
Given that the clinical population undergoing LT and the treatment of these patients has changed significantly in recent years, it stands to reason that antiquated data may not be generalizable to modern practice. The aim of this multicenter cohort study is to provide contemporary figures for the cumulative incidences of solid and hematological DNM after LT.
2Patients and methodsWe designed a retrospective cohort study including all adult patients undergoing LT between 2000 and 2015 in three transplant centers (Umberto I University Hospital, Fondazione Policlinico Universitario Agostino Gemelli IRCSS, Fondazione Policlinico Tor Vergata) in Rome, Italy. All patients were followed periodically, and information were collected accurately for any DNM.
Exclusion criteria were:
- a)
re-transplantation;
- b)
multi-organ transplantation;
- c)
pediatric (<18 years) transplantation;
- d)
patients with a post-transplant follow-up shorter than six months, the latter because cancer is a time-dependent event and these patients were not at risk of developing DNM.
For each transplanted patient the following variables were collected: date of transplantation, age at transplant, gender, indication for LT, any alcohol relapse/slip after LT, smoking pattern, immunosuppressive therapy, post-LT Cytomegalovirus (CMV) infection, post-LT Epstein Barr Virus (EBV) infection, pre-LT history of cancer, patient survival, and causes of death. Post-LT EBV and CMV infection were diagnosed by CMV DNA and EBV DNA quantitative analysis. Protocols for EBV viremia surveillance were introduced in 2012–2013 and foresee EBV DNA viremia every 2–4 weeks in the first 3 months, monthly until 6 months post-transplantation and then every 3 months for the rest of the first year in patients with donor seropositive/recipient seronegative status.
For cases of DNM, data collected included the timing, presence of a surveillance program, characteristics of clinical presentation, staging at diagnosis, treatment and outcomes.
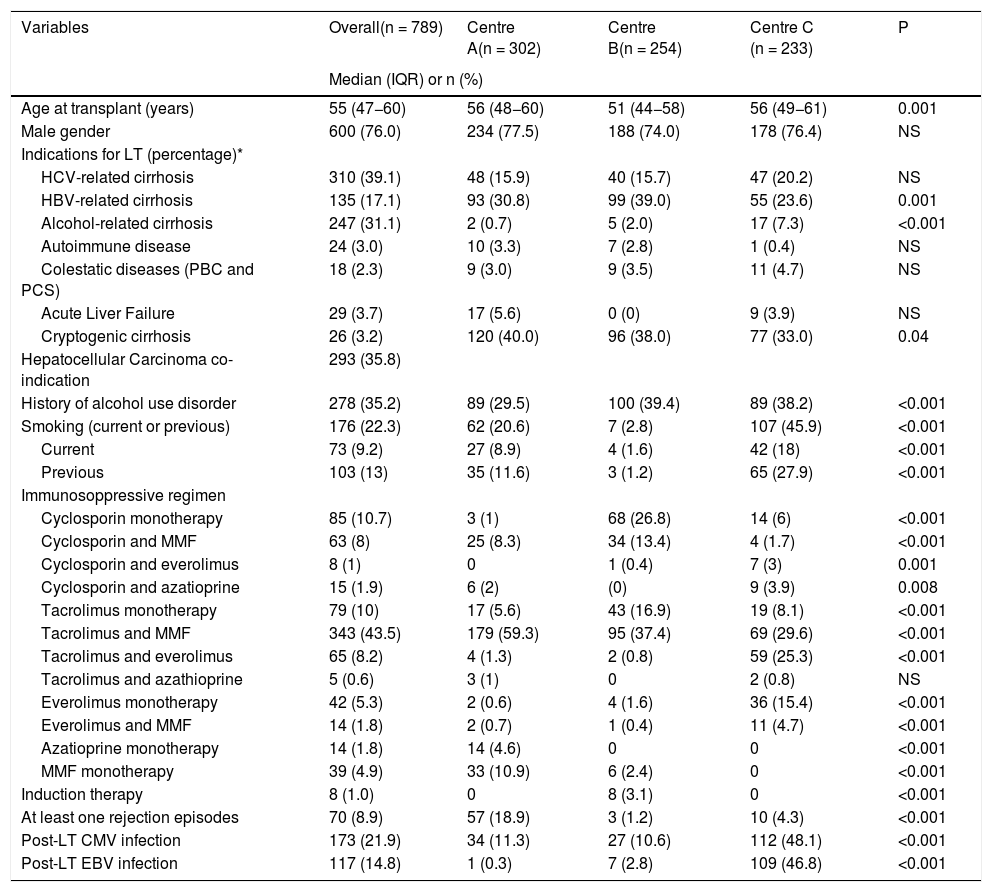
2.1ImmunosuppressionThe immunosuppressive protocols depended on the year of transplantation and choices of the center. Over the years, 12 different primary immunosuppression regimens had been combined, as shown in Table 1. The most common regimens included a calcineurin inhibitor (cyclosporin or tacrolimus) and mycophenolate mofetil. About 7% of patients received everolimus-based immunosuppression. Additionally, a minority of the LT recipients, especially those with concomitant severe renal failure, received induction therapy with an anti-thymocyte globulin or monoclonal antibodies directed against interleukin 2 receptors. Based on clinical judgment, patients with an acute cellular rejection were treated with 3 intravenous boluses of methylprednisolone (500 mg). Steroid-resistant rejection was treated with anti-thymocyte globulin.
Patients’ characteristics in the three Centers.
| Variables | Overall(n = 789) | Centre A(n = 302) | Centre B(n = 254) | Centre C (n = 233) | P |
|---|---|---|---|---|---|
| Median (IQR) or n (%) | |||||
| Age at transplant (years) | 55 (47−60) | 56 (48−60) | 51 (44−58) | 56 (49−61) | 0.001 |
| Male gender | 600 (76.0) | 234 (77.5) | 188 (74.0) | 178 (76.4) | NS |
| Indications for LT (percentage)* | |||||
| HCV-related cirrhosis | 310 (39.1) | 48 (15.9) | 40 (15.7) | 47 (20.2) | NS |
| HBV-related cirrhosis | 135 (17.1) | 93 (30.8) | 99 (39.0) | 55 (23.6) | 0.001 |
| Alcohol-related cirrhosis | 247 (31.1) | 2 (0.7) | 5 (2.0) | 17 (7.3) | <0.001 |
| Autoimmune disease | 24 (3.0) | 10 (3.3) | 7 (2.8) | 1 (0.4) | NS |
| Colestatic diseases (PBC and PCS) | 18 (2.3) | 9 (3.0) | 9 (3.5) | 11 (4.7) | NS |
| Acute Liver Failure | 29 (3.7) | 17 (5.6) | 0 (0) | 9 (3.9) | NS |
| Cryptogenic cirrhosis | 26 (3.2) | 120 (40.0) | 96 (38.0) | 77 (33.0) | 0.04 |
| Hepatocellular Carcinoma co-indication | 293 (35.8) | ||||
| History of alcohol use disorder | 278 (35.2) | 89 (29.5) | 100 (39.4) | 89 (38.2) | <0.001 |
| Smoking (current or previous) | 176 (22.3) | 62 (20.6) | 7 (2.8) | 107 (45.9) | <0.001 |
| Current | 73 (9.2) | 27 (8.9) | 4 (1.6) | 42 (18) | <0.001 |
| Previous | 103 (13) | 35 (11.6) | 3 (1.2) | 65 (27.9) | <0.001 |
| Immunosoppressive regimen | |||||
| Cyclosporin monotherapy | 85 (10.7) | 3 (1) | 68 (26.8) | 14 (6) | <0.001 |
| Cyclosporin and MMF | 63 (8) | 25 (8.3) | 34 (13.4) | 4 (1.7) | <0.001 |
| Cyclosporin and everolimus | 8 (1) | 0 | 1 (0.4) | 7 (3) | 0.001 |
| Cyclosporin and azatioprine | 15 (1.9) | 6 (2) | (0) | 9 (3.9) | 0.008 |
| Tacrolimus monotherapy | 79 (10) | 17 (5.6) | 43 (16.9) | 19 (8.1) | <0.001 |
| Tacrolimus and MMF | 343 (43.5) | 179 (59.3) | 95 (37.4) | 69 (29.6) | <0.001 |
| Tacrolimus and everolimus | 65 (8.2) | 4 (1.3) | 2 (0.8) | 59 (25.3) | <0.001 |
| Tacrolimus and azathioprine | 5 (0.6) | 3 (1) | 0 | 2 (0.8) | NS |
| Everolimus monotherapy | 42 (5.3) | 2 (0.6) | 4 (1.6) | 36 (15.4) | <0.001 |
| Everolimus and MMF | 14 (1.8) | 2 (0.7) | 1 (0.4) | 11 (4.7) | <0.001 |
| Azatioprine monotherapy | 14 (1.8) | 14 (4.6) | 0 | 0 | <0.001 |
| MMF monotherapy | 39 (4.9) | 33 (10.9) | 6 (2.4) | 0 | <0.001 |
| Induction therapy | 8 (1.0) | 0 | 8 (3.1) | 0 | <0.001 |
| At least one rejection episodes | 70 (8.9) | 57 (18.9) | 3 (1.2) | 10 (4.3) | <0.001 |
| Post-LT CMV infection | 173 (21.9) | 34 (11.3) | 27 (10.6) | 112 (48.1) | <0.001 |
| Post-LT EBV infection | 117 (14.8) | 1 (0.3) | 7 (2.8) | 109 (46.8) | <0.001 |
Missing values: Age = 5, Immunosoppressive regimen = 17.
Abbreviations: IQR, interquartile ranges; LT, liver transplantation; HCV, hepatitis C virus; HBV, hepatitis B virus; MMF, mycophenolate mofetil; CMV, cytomegalovirus, EBV Epstein Barr Virus, PBC Primary Biliary Cholangitis, PSC Primary Sclerosing Cholangitis.
Before transplant all candidates (except those with acute liver failure) underwent thorough tumor screening including chest radiography, abdominal computed tomography, gastroscopy, colonoscopy, mammography, Papanicolaou smear, transvaginal pelvic ultrasound, and prostate-specific antigen assessment.
Post-LT cancer surveillance strategies (Supplementary Table 1) at each of the three centers were similar. Strategies included annual mammography starting at the age of 40 years, annual Papanicolaou smear, annual prostate-specific antigen starting at the age of 50 years and colonoscopy every 5 years (or annually in patients with primary sclerosing cholangitis and inflammatory bowel disease).
A screening protocol for skin cancers (annual dermatological exam) was adopted, identifying a small number of early melanomas (all Breslow depth <1 mm) and other skin cancers. These were not included in our analysis as these tumors have a very limited impact on mortality in LT patients [15]. In our cohort we had no cases of metastatic skin cancer of any type.
2.3Statistical analysisContinuous variables were reported as medians and interquartile ranges (IQR). Categorical variables were reported as numbers and percentages. We used the maximum likelihood estimation method for managing missing data: in each variable, less than 10% of the data was initially missing. Kruskal–Wallis and chi-squared Pearson tests were used for comparisons of continuous and categorical variables, respectively.
Post-LT HCC recurrence was not considered as an event in the present study. Consequently, patients that developed HCC were censored at the last follow-up date. Survival probabilities were estimated using the Kaplan–Meier method.
Cox regression models were constructed to identify risk factors for post-LT DNM and post-DNM-related death. All covariates showing a p-value <0.2 in univariate analysis were used for the construction of the multivariable Cox regression analysis. Hazard ratios (HR) and 95% confidence intervals (95%CI) were reported.
Cumulative incidence was calculated at 5, 10 and 15 years from LT by Kaplan–Meyer analysis.
Variables with a p-value <0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA).
3Results3.1Study populationBetween 2000 and 2015, 968 patients were transplanted at the three transplant centers. 22 patients were excluded because of re-transplantation, 7 patients because of multi-organ transplantation, 11 patients because of pediatric transplantation and 139 patients because they had a post-transplant follow-up shorter than six months. The study population included 789 patients.
Within out cohort the median age was 55 years (range 18–76), and 600 (76%) patients were male. The most common indications for LT were HCV-related cirrhosis (39%) and alcohol-related cirrhosis (31%), while 35% of patients underwent LT because of HCC. History of alcohol use disorder and smoking was present in 35% and 22% of patients, respectively. The commonest immunosuppressive regimes were tacrolimus and mycophenolate mofetil (43%), tacrolimus monotherapy (10%) and cyclosporine monotherapy (10%). Post-LT Cytomegalovirus (CMV) and Epstein Barr Virus (EBV) infection were reported for 22% and 15% of patients, respectively. Differences between the three centers are presented in table 1 and involved indications for liver transplantation, alcohol and smoking habits, and immunosuppressive regimes.
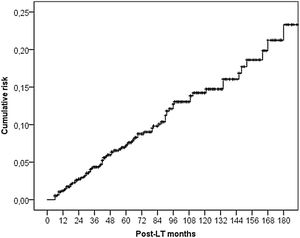
3.2DNM incidenceSixty-seven (5.5%) patients developed a non-cutaneous DNM (Table 2). The cumulative incidence of non-cutaneous DNM was 6.2% at 5-years, 11.6% at 10-years and 16.3% at 15-years (Fig. 1). Median follow-up was 81 months (IQR: 38–124). Post-Transplant Lymphoproliferative Disorders (PTLD) accounted for 9 cases, their cumulative incidence was observed to be 1.0% at 5-years, 1.6% at 10-years and 2.2% at 15-years. 3 of these patients developed EBV infection post-LT. Overall Solid Organ Tumors (SOT) accounted for the majority, representing 57 cases, with a cumulative incidence of 5.3% at 5-years, 10.3% at 10-years and 14.4% at 15-years. The most frequently observed classifications of SOT involved lung and head & neck tumors. Lung tumors accounted for 11 cases (7 non-small cell lung cancers, 3 small cell cancers, 1 neuroendocrine tumor) with a 5-years cumulative incidence of 1.0%; 45% of them occurred in patients with a history of smoking. Head & neck tumors, specifically squamous cell carcinomas, were diagnosed in 11 patients (8 larynx, 2 tongue, 1 pharynx), with a 5-years cumulative incidence of 1.3%; 50% of them occurred in patients with a history of alcohol and/or smoking. No statistical differences in the incidences of DNM were observed among the three centers.
Cumulative incidence of De Novo Malignancy by site.
| Site | N° case(on 789 LT) | Cumulative incidence5 years after LT(n° at risk 465) | Cumulative incidence10 years after LT(n° at risk 209) | Cumulative incidence15 years after LT(n° at risk 48) |
|---|---|---|---|---|
| Lung | 11 | 1.0% | 2.5% | 2.5% |
| Head and neck | 11 | 1.3% | 1.9% | 1.9% |
| PTLD | 9 | 1.0% | 1.6% | 2.2% |
| Breast | 5 | 0.4% | 0.7% | 2.2% |
| Esophagus | 4 | 0.4% | 0.4% | 1.9% |
| Prostate | 4 | 0.2% | 1.1% | 1.1% |
| Pancreas | 4 | 0.4% | 0.7% | 1.4% |
| Stomach | 3 | 0.4% | 0.7% | 0.7% |
| Colon | 3 | 0.2% | 0.8% | 0.8% |
| Kaposi | 3 | 0.2% | 0.5% | 1.2% |
| Others* | 10 | |||
| Solid organ tumor | 57 | 5.3% | 10.3% | 14.4% |
| All non-cutaneous DNM | 67 | 6.2% | 11.6% | 16.3% |
PTLD Post-Transplant Lymphoproliferative Disorder; DNM De Novo Malignancy; LT Liver Transplantation.
Patients with non-cutaneous DNM were mainly male (n = 52, 78%) presenting at a median age at diagnosis of 59 years (IQR: 51–63). The median interval between LT and the diagnosis of the tumor was 47 months (IQR: 22–94). The interval between LT and DNM occurrence was different according to the type of tumor, with a median value of 33 months (IQR: 9–65) for PTLD, 43 months (IQR: 31–65) for head & neck tumors, and 59 months (IQR: 14–96) for lung tumors. Only one case of DNM was observed among 29 patients transplanted for acute liver failure and was a case of PTLD occurring 9 years after LT.
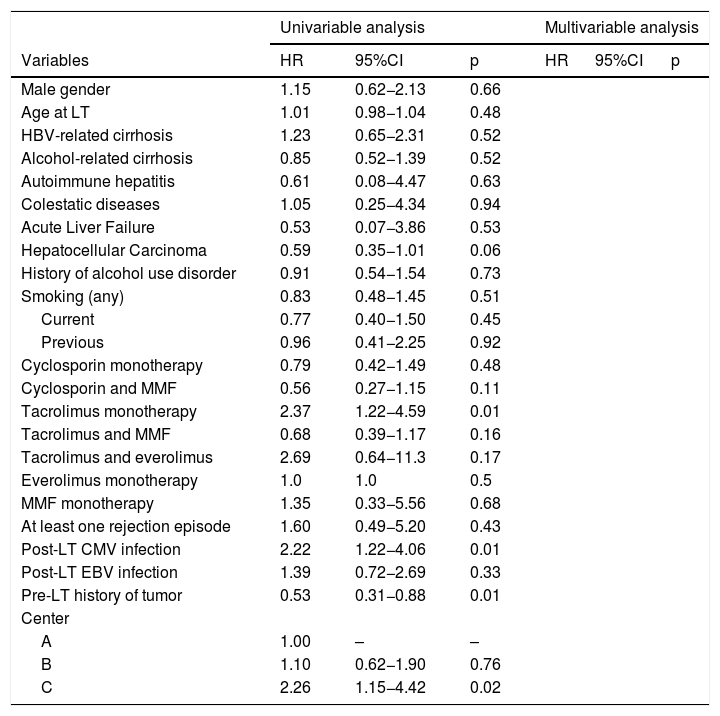
3.3Risk factorsRisk factors associated with the development of SOT are reported in Table 3. In univariable analysis, tacrolimus monotherapy (HR 2.37; p = 0.01), post-LT CMV infection (HR 2.22; p = 0.01), pre-LT history of tumor (HR = 0.53; p = 0.02) were significantly associated with SOT development. These isolated associations did not reach significance in multivariable analysis.
Cox regression analysis for the risk factors of the post-LT SOT.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95%CI | p | HR | 95%CI | p |
| Male gender | 1.15 | 0.62−2.13 | 0.66 | |||
| Age at LT | 1.01 | 0.98−1.04 | 0.48 | |||
| HBV-related cirrhosis | 1.23 | 0.65−2.31 | 0.52 | |||
| Alcohol-related cirrhosis | 0.85 | 0.52−1.39 | 0.52 | |||
| Autoimmune hepatitis | 0.61 | 0.08−4.47 | 0.63 | |||
| Colestatic diseases | 1.05 | 0.25−4.34 | 0.94 | |||
| Acute Liver Failure | 0.53 | 0.07−3.86 | 0.53 | |||
| Hepatocellular Carcinoma | 0.59 | 0.35−1.01 | 0.06 | |||
| History of alcohol use disorder | 0.91 | 0.54−1.54 | 0.73 | |||
| Smoking (any) | 0.83 | 0.48−1.45 | 0.51 | |||
| Current | 0.77 | 0.40−1.50 | 0.45 | |||
| Previous | 0.96 | 0.41−2.25 | 0.92 | |||
| Cyclosporin monotherapy | 0.79 | 0.42−1.49 | 0.48 | |||
| Cyclosporin and MMF | 0.56 | 0.27−1.15 | 0.11 | |||
| Tacrolimus monotherapy | 2.37 | 1.22−4.59 | 0.01 | |||
| Tacrolimus and MMF | 0.68 | 0.39−1.17 | 0.16 | |||
| Tacrolimus and everolimus | 2.69 | 0.64−11.3 | 0.17 | |||
| Everolimus monotherapy | 1.0 | 1.0 | 0.5 | |||
| MMF monotherapy | 1.35 | 0.33−5.56 | 0.68 | |||
| At least one rejection episode | 1.60 | 0.49−5.20 | 0.43 | |||
| Post-LT CMV infection | 2.22 | 1.22−4.06 | 0.01 | |||
| Post-LT EBV infection | 1.39 | 0.72−2.69 | 0.33 | |||
| Pre-LT history of tumor | 0.53 | 0.31−0.88 | 0.01 | |||
| Center | ||||||
| A | 1.00 | – | – | |||
| B | 1.10 | 0.62−1.90 | 0.76 | |||
| C | 2.26 | 1.15−4.42 | 0.02 | |||
Abbreviations: HR, hazard ratio; CI, confidence intervals; LT, liver transplantation; HCV, hepatitis C virus; HBV, hepatitis B virus; MMF, mycophenolate mofetil; CMV, cytomegalovirus; EBV, Epstein Barr Virus.
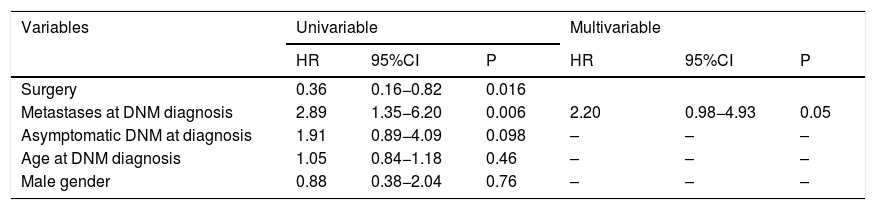
Table 4 shows SOT-related prognostic factors. As for the multivariable Cox regression analysis results, the presence of metastases at diagnosis (HR = 2.20; P = 0.05) was a border-line risk factor for mortality.
Uni- and multivariate Cox regression analysis for mortality after SOT onset.
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Surgery | 0.36 | 0.16−0.82 | 0.016 | |||
| Metastases at DNM diagnosis | 2.89 | 1.35−6.20 | 0.006 | 2.20 | 0.98−4.93 | 0.05 |
| Asymptomatic DNM at diagnosis | 1.91 | 0.89−4.09 | 0.098 | – | – | – |
| Age at DNM diagnosis | 1.05 | 0.84−1.18 | 0.46 | – | – | – |
| Male gender | 0.88 | 0.38−2.04 | 0.76 | – | – | – |
Abbreviations: HR, hazard ratio; CI, confidence intervals; DNM, de-novo malignancy; LT, liver transplantation.
After splitting the population into two groups according to the date of transplantation (2000–2009 vs. 2010–2015), several differences were observed (Supplementary Table 1). In the more recent period, older patients were transplanted (median age 56 vs. 53 years; p = 0.001). Indications for LT remained similar, although more patients received transplants for cryptogenic cirrhosis (5.1 vs. 2.1%; p = 0.03).
As for smoking history and alcohol use disorder, more cases were reported in recent years. However, such a result could have been influenced by more accurate data collection in the second period. Similarly, the greater number of post-LT CMV and EBV infections identified in this period could be related to such a phenomenon.
Immunosuppressive regimens significantly changed between the first and second era, with a progressive decline in the use of cyclosporine (cyclosporine monotherapy: 17.5 vs. 0.6%, p < 0.0001; cyclosporine + MMF: 12.5 vs. 1.3%, p < 0.0001), an increase in the use of tacrolimus (tacrolimus + MMF: 32.1 vs. 60.4; p < 0.0001), and introduction of everolimus (tacrolimus + everolimus: 2.5 vs. 16.8; p < 0.0001). Interestingly, the percentage of patients reporting a DNM within 5 years from LT was similar (4.4 vs. 6.0%; p = 0.33).
4DiscussionDespite major advances in all aspects of pre- and post-operative management of patients with end-stage liver disease undergoing LT, malignancies are reported to be the most common cause of death in patients surviving at least one year [2,3,16]. For this reason, updated data concerning the incidence, risk factors and prognosis of DNM are important to target specific surveillance protocols in the post-transplant setting. Currently, surveillance protocols for cancer in patients that underwent LT were based on expert opinions, often derived from recommendations for the general population, and not always applied appropriately [6,17,18]. Furthermore, it may also happen that patients, underestimating the risks connected with the chronic immunosuppression, become less compliant with screening measures as time passes following transplantation.
In the present analysis, the incidence of DNM was not different among the three centers despite some differences in the immunosuppressive regimens. However, each center avoided high level of immunosuppressive drugs. We failed to demonstrate a role for immunosuppressive regimens or moderate target dosages in the development of DNM or PTLD.
The incidence of DNM after LT in our study was higher than that found in the general population [19]. In particular, the annual incidence per 100,000 of non-cutaneous malignancy in 55 year old males in the cancer registry of the same geographical area was about one third lower than that of our transplant population (7198 vs 10,866). The difference was even more pronounced when comparing the most frequent types of cancer in our cohort: lung (1194 vs 250), hematologic malignancy (646 vs 220) and head & neck tumors (488 vs 190).
Our cumulative incidences of non-cutaneous DNM (6.2% at 5-years and 11.6% at 10-years) are comparable with older publications [4,15,20–24]. Specifically, a German single-centre study analysing the period 1988–2006 reported cumulative incidence of 7% at 5-years and 12.9% at 10-years [4]. A Dutch single-centre study analysing the period 1986–2007 reported a cumulative incidence of 5.4% at 5-years and 10.8% at 10-years [15]. Similar data (cumulative incidence rates of 4% at 5-years and 9% at 10-years) came also from Nordic LT Registry analyzing the period 1982–2013 [20].
Notably however, the incidences of each classification of DNM in our series were different than reported elsewhere. Firstly, we observed lower cumulative incidences for PTLD compared to those reported in the literature. We report incidences of 1.0% at 5-years and 1.6% at 10-years whereas rates of 1.6% at 5-years and 2.3% at 10-years were seen in the German study [4], 2.8% at 5-years and 6.4% at 10-years in the Dutch study [15] and even higher in older series [21,24,25]. However, the study is underpowered to make definitive assumptions about PTLD as a separate entity. The reasons for these differences surpass the purposes of our study, but current practice of routine monitoring of EBV-DNA viremia and pre-emptive reduction of immunosuppression in patients at high-risk of EBV-DNA viremia may play a role [26]. Unfortunately, a screening protocol for these tumors presently does not exist.
In our cohort, the most common SOT were lung and head & neck tumors, which are traditionally linked to smoking and alcohol habit [27]. An Italian multicenter case-control study confirmed a significant impact on mortality for both cancers; HR = 37.13 for lung cancer and 4.65 for head & neck cancers [28]. In this multi-centre series the number of lung and head-neck tumors was fortunately low. Nevertheless, regular counseling on smoking cessation should be mandatory for all LT patients both in the short and in the long term and a cessation should start at the time of evaluation for LT listing. Herrero et al. demonstrated that smoking withdrawal after liver transplantation may have a protective effect against the development of cancer [29]. Currently, surveillance protocols for these tumor subtypes in the setting of LT do not exist and risk stratification of LT patients seems the most promising approach to implement effective and applicable screening protocols. The screening proposal of Burra et al. includes yearly thoracic CT scanning in active smokers, and ears, nose and throat examination in patients transplanted for alcoholic liver disease, especially with contemporaneous smoking habit [17]. Future research should prospectively validate these types of surveillance program.
This study has some limitations. Firstly, the study is retrospective and examines a 15-year period. These shortcomings limited our possibility to systematically collect the immunosuppressive blood levels to assess whether adverse effects were dose-dependent. Thus, a safer immunosuppressive threshold could not be determined. Moreover, the switch from dual to mono-therapy during this period was not investigated due to the difficulties in collecting regime changes during a 15-year period.
Second, both smoking and alcohol use disorder were analyzed using less granular, often dichotomous, variables, due to the difficulty in collecting daily usage and duration of abuse for all study participants. We also could not collect smoking and alcohol status before and after transplant separately. Lastly, some interesting variables like diabetes mellitus and metabolic syndrome before or after LT, and the episodes of acute rejection were not considered in the analysis, due to missing data in the database. Despite these limitations the differences in the immunosuppressive regiments among centers constitute a strength and allow a potential stratification of results.
In conclusion, our study demonstrates that lung and head & neck tumors are the most frequent SOT after LT. Our data support the development of surveillance programs for these tumors as a priority. Immunosuppressive regimes do not appear to be associated with different incidences of DNM.
Financial supportNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Dr. Christopher Oddy (Epsom & St Helier University Hospitals NHS Trust, United Kingdom) for proof-reading the text.