Background. One established model to induce hepatic preneoplasia (HP) (DEN 150) uses diethylnitrosamine (DEN) as initiator agent and 2-acetylaminofluorene (2-AAF) as a promoter drug. In addition, both chemicals cause liver cholestasis and fibrosis. Aim. We compared DEN 150 model with another adapted by us, DEN 200 to simplify the first one and to evaluate the effectiveness of both treatments to induce HP in rats.
Material and methods. Male Wistar rats were divided in 3 groups: controls; DEN 150 (rats received 2 doses of DEN, 150 mg/kg body weight, 2 weeks apart, and then 2-AAF, 20 mg/kg body weight, 4 doses per week during 3 weeks); and DEN 200 (rats received a single dose of DEN 200 mg/kg body weight, and 2 weeks apart 2-AAF, 20 mg/kg body weight, 2 doses per week during 3 weeks). Four hepatic enzymes, prothrombin time percentage, the number of bile ductules, total collagen amount, the number of altered hepatic foci (AHF) per liver and the percentage of liver occupied by foci were analyzed.
Results. There were no differences in the number of AHF per liver between treated groups. Rats from DEN 200 group showed a significant diminution in the volume of liver occupied by foci. DEN 200 group had no fibrosis and better hemostatic conditions than DEN 150 group. Both groups developed cholestasis. Conclusion. In conclusion, both protocols are good alternatives to induce HP in rats and the new protocol proposed is an effective and a simple methodology to provide subclinic states of liver cancer.
Several models in rat liver have been developed to study multistage carcinogenesis, including the Solt-Farber resistant hepatocyte model. The system consists of a single dose of the genotoxic carcinogen diethylnitrosamine (DEN), short-term dietary exposure to 2-acetylaminofluorene (2-AAF), sufficient to suppress the growth of virtually all normal hepatocytes, and partial hepatectomy (PH) to trigger the growth of DEN-altered hepatocytes not suppressed by 2-AAF.1 DEN produces DNA base modifications and DNA strand-breaks that conduct to altered (initiated) cells containing an irreversible genetic change.2 The subsequent stage of promotion involves the selective clonal expansion of these initiated cells into foci of altered hepatocytes (AHF).3
Alvarez, et al. developed a 2-phase model (initiation-promotion) of rat liver preneoplasia based on Solt-Farber model in which PH was eliminated. Authors used two necrogenic doses of DEN two weeks apart and four doses per week of 2-AAF by gavage during three weeks. Necrogenic doses of DEN cause massive hepatic necrosis followed by regeneration and would be expected to cause not only increased gene expression related to regeneration but also increased expression related to oncogene mutation.4 In this model, AHFs emerge in livers of treated rats at the end of the six-week treatment. This two-stage or initiation-promotion model mimics the latent period of human hepatocarcinogenesis and is considered a useful tool to study molecular mechanisms occurring during the early stages of liver cancer.
AimThe aims of the present work were:
- •
To simplify the model developed by Alvarez, et al., thus reducing animal manipulation and
- •
To evaluate the effectiveness of both treatments to induce hepatic preneoplasia in rats by studying early stage parameters of hepatic carcinogenesis.
Male adult Wistar rats (300-350 g body weight) were maintained two per cage on a constant 12 h light/dark cycle under controlled temperature and humidity conditions. They had free access to tap water. They were fed with standard rat pellets ad libitum. All the experimental protocols were performed according to the NIH Guide for the Care and Use of Laboratory Animals5 and approved by the Guide for the Care and Use of Laboratoy Animals Committee, Facultad de Ciencias Bioquímicas y Farmacéuticas, Universidad Nacional de Rosario, Resolution No 6109/012.
Chemicals and reagentsDEN and 2-AAF were obtained from Sigma Chemical Co. (St Louis, MO). Rabbit polyclonal anti-GST3/GST-pi antibody was purchased from Abcam® (AB106268, Boston, USA). Reagents for enzymatic determinations were provided by Wiener Laboratories S.A.I.C. (Rosario, Argentina). For prothrombin time determination Dade® Innovin® reagent (Siemens, USA) was used.
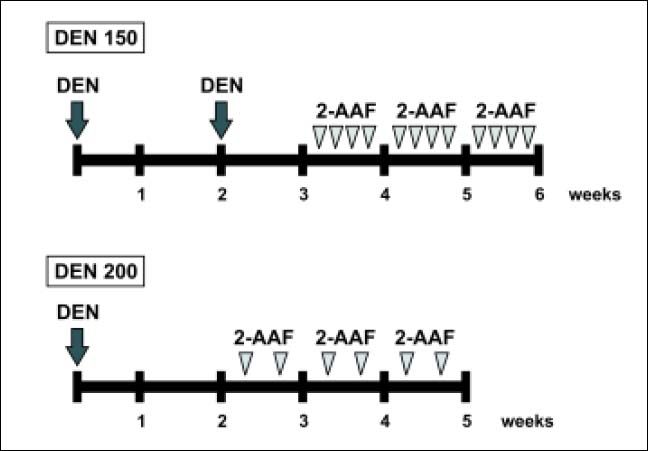
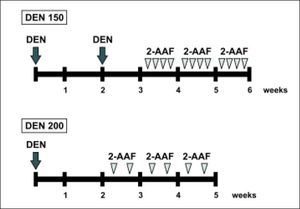
Experimental designThe animals were divided into 3 groups of 4-6 rats each. Animals of group 1 were used as normal controls and received the vehicles of the drugs. Rats from groups 2 and 3 were subjected to a 2-phase model of rat hepatocarcinogenesis. In group 2 (DEN 150 group), the initiation stage was performed by the administration of 2 intraperitoneal necrogenic doses of DEN (150 mg/kg body weight) two weeks apart. Administration of 2-AAF was performed one week after the last injection of DEN. The 2-AAF was dissolved in dimethyl sulfoxide and then suspended in corn oil to a final concentration of 8 mg/ mL. Rats received 20 mg/kg body weight of 2-AAF by gavage for 4 consecutive days per week during 3 weeks.4 Animals from group 3 (DEN 200 group) were subjected to a 2-phase model of rat hepatocarcinogenesis adapted from the one of Alvarez, et al. In this protocol, a unique dose of DEN (200 mg/kg body weight) was administrated intraperitoneally as an initiation stage and two weeks later 20 mg/kg body weight of 2-AAF was given to the animals 2 days (one day apart) per week during 3 weeks. A scheme of the experimental protocols is shown in figure 1. To avoid variations due to circadian rhythm, all animals were euthanized between 9 and 11 AM.
Male Wistar rats were subjected to the initiation-promotion protocols of chemical carcinogenesis. DEN was used as the initiator, administered intraperitoneally, and 2-AAF was administered by gavage as the promotion agent. DEN 150 group received 2 doses of DEN (150 mg/kg body weight), two weeks apart. One week after the last injection of DEN, 2-AAF (20 mg/kg body weight) was administered four days per week during three weeks. DEN 200 group received a single dose of DEN (200 mg/kg body weight) and two weeks later 2-AAF (20 mg/kg body weight) was given to the animals 2 days per week for 3 weeks. n = 4-6 animals per experimental group.
At the end of the treatment, rats were anesthetized with ketamine-xilazine (70 mg/kg body weight and 2.1 mg/kg body weight, respectively) and bled through cardiac puncture. Livers were removed for histological and immunohistochemical analysis. Alkaline phosphatase (ALP), alanine and aspartate aminotransferases (ALT/AST, respectively) and hepatic cholinesterase (ChE) were determined spectrophotometrically at 25 oC using fresh serum. For prothrombin time (PT) measurement, 2.5 mL of blood was drawn into a test tube containing 0.25 mL sodium citrate.6
Histological and immunohistochemical proceduresPieces of liver tissue from all groups were fixed in 10% v/v formaldehyde and histological processed for paraffin embedded. In sections of 4 μm thickness, an immunohistochemical staining was performed. Briefly, deparaffinized tissue sections were treated with 3% H2O2 in methanol for 10 min to remove endogenous peroxidase, and then they were micro-waved in a 10 mM citrate buffer solution for 10 min at 96 °C to perform antigen retrieval. Normal serum was applied to the slides to block nonspecific binding, and the slides were incubated with rabbit polyclonal antibody against the placental form of rat Glutathione S-transferase (rGST-P, 1/250) (Abcam, catalogue AB106268) overnight at 4 oC. rGST-P is the most effective marker of hepatic preneoplasia in rats.7 The slides were incubated with biotinilated goat anti-rabbit secondary antibody and then with horseradish-peroxidase-conjugated streptavidin (HRP CytoScan Detection Kit-Cell Marque, catalogue CMD302). The signals were detected with DAB Sustrate Kit (Cell Marque, catalogue 957D-20) followed by counsterstaining with hematoxylin.
AHF quantificationAfter immunohistochemical procedures, a representative number of field sections (usually 1-1.5 cm2 of tissue per animal) from DEN 150 and DEN 200 groups were collected using a digital camera (Olympus D-360, Tokyo, Japan) attached to a light field microscopy (Olympus, U-MDOB model). Images were quantified using analysis software (ImageJ, U.S. National Institutes of Health, Bethesda, Maryland, USA). The number of AHF per liver and the percentage of liver occupied by foci were calculated according to the modified Saltykov’s method8 (n = 4 rats per group).
Hepatic collagen evaluationLiver fibrosis was evaluated by digital image analysis on sections stained with 1 % Direct Red 80/ picric acid. This semi-quantitative technique allows the estimation of total amount of hepatic collagen using several microphotographs slices obtained from each liver slice. For semi quantification Adobe® Photoshop 6.0 (Adobe Systems Inc.) and Scion Image version Beta 4.0.2 (Scion Corporation) software were used. Three animals per group were used. Fifteen pictures from each animal were obtained with a light field microscopy (40X objective), equipped with a digital camera. Pictures were randomly obtained taking care not to dwell on the same area twice (portal areas were no discarded). Briefly, on the Adobe® Photoshop software the red colour of Direct Red 80 was selected. The area occupied by collagen was extracted from the remainder of the picture taking care to extract the highest amount of collagen fibers. Then, extracted area was painted black and saved as tiff format to be quantified by Scion Image software. The area of the picture and its resolution were standardized (28.346 pixel/cm). On the Scion software the collagen area was calculated. This area was divided by picture area and the density of collagen was calculated. Results were expressed as cm2 occupied by collagen/cm2 of the picture.9
Bile ductules proliferationTo analyze ductular proliferation, liver sections from 3 rats of each group were stained with H&E. Fifty microscopic fields from each specimen were randomly chosen (portal areas were no discarded) to assess the number of bile ductules. Ductular proliferation was defined as the number of bile ductules per microscopic field (400X).10
Statistical analysisData were statistically analyzed by one way ANOVA followed by post hoc analysis using Tukey multiple comparison test. Because ANOVA requirements were not completely satisfied for hepatic collagen amount, number of bile ductules, GOT activity and percentage of prothrombin time, a nonparametric Kruskal-Wallis test was used followed by Dunn’s comparisons. The number of AHF per liver and the percentage of liver occupied by foci were analyzed by Student’s t-test. The results were expressed as means ± SE. A value of p ≤ 0.05 was considered to be statistically significant.
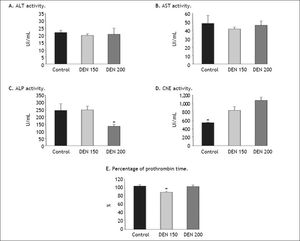
ResultsEnzymes activities and PT determinationWe measured transaminases (AST and ALT) which are useful biomarkers of liver injury in a patient with some degree of intact liver function. Other tests were ChE, ALP and PT determination.11 ChE is an enzyme which shows low values during the course of liver diseases, especially when a great amount of hepatic cells is damaged and the ability of the liver to synthesize new proteins is diminished.12 Results are shown in figure 2.
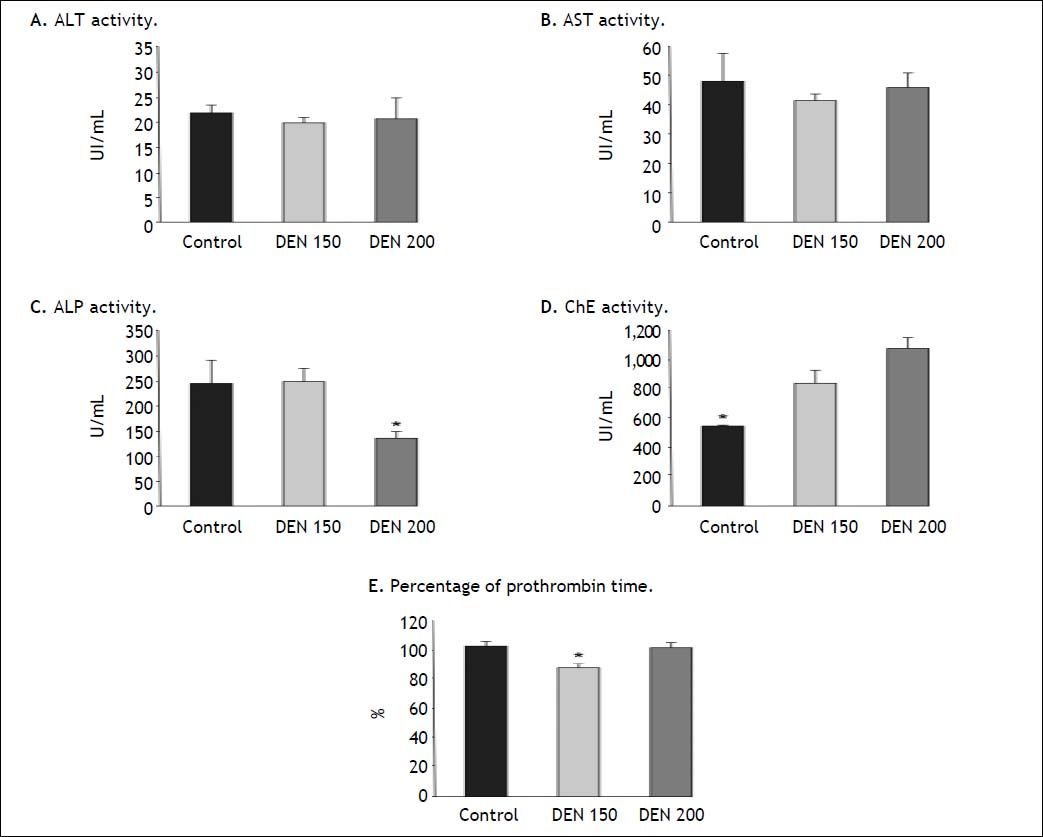
Hepatic enzymes activities and percentage of prothrombin time determinations. A. ALT and B AST activities did not show significant differences between groups, n = 4 for each enzyme. C. ALP activity was significantly lower in DEN 200 group compared to control and DEN 150 groups, n = 4. D. ChE activity was significantly lower for control group than for treated ones, n = 4. E. Percentage of prothrombin time was significantly lower in DEN 150 group, n = 4. Each bar represents the mean ± SE, * p ≤ 0.05.
ALT and AST activities had no differences between groups (ALT, controls: 21.6 ±1.9 UI/mL; DEN 150: 19.7 ± 1.2 UI/mL; DEN 200: 20.5±4.2 UI/mL. AST, controls: 47.75 ± 9.3 UI/mL; DEN 150: 41.3 ± 2.2 UI/mL; DEN 200: 45.7 ± 5.2 UI/ mL). Rats from DEN 200 group had the lowest ALP values while no statistical differences were obtained for ALP values between DEN 150 and control group (controls: 243.3 ± 45.0 UI/mL; DEN 150: 245.3 ± 28.4 UI/mL; DEN 200: 133.2 ± 17.1 UI/mL). Control group presented the lowest ChE activity and no differences in this parameter were observed between treated groups (controls: 545.5 ± 8.7 UI/mL; DEN 150: 830.7 ± 91.6 UI/mL; DEN 200: 1,065.5 ± 77.2 UI/mL). DEN 150 group had the lowest percentage of PT (controls: 103.0 ± 2.5 %; DEN 150: 87.7 ± 2.7 %; DEN 200: 102.0 ± 3.6 %). From these results we could infer that livers from DEN 200 group had better hemostatic conditions than DEN 150 group while hepatic enzymatic activities were similar between treated groups except for ALP values. Livers from treated groups conserved, to a great extent, their functionality.
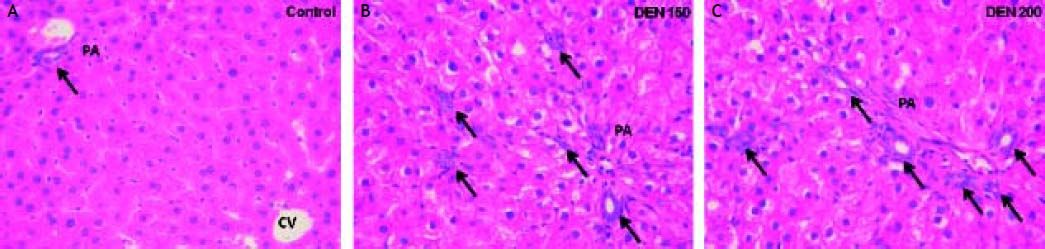
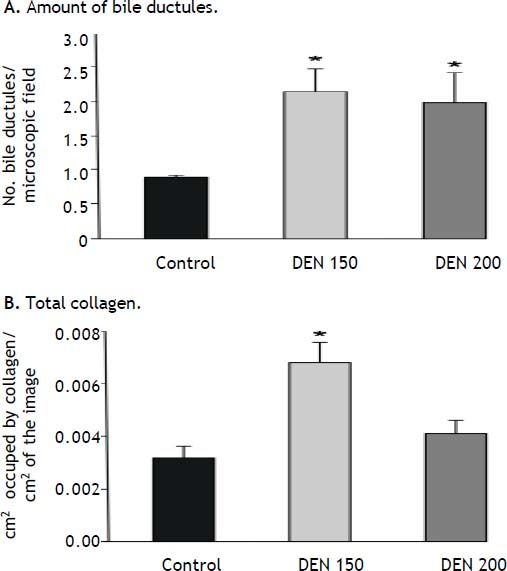
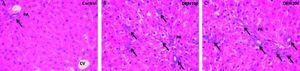
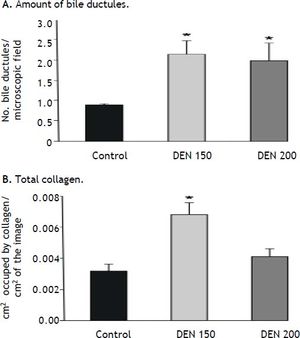
Bile ductules proliferation and total collagen estimationDEN 150 and DEN 200 groups had significantly more bile ductules than controls (controls: 0.90 ± 0.03; DEN 150: 2.13 ± 0.34; DEN 200: 1.97 ± 0.45). Many bile ductules could be found within the hepatic parenchyma in livers from DEN groups. The number of ductules in portal areas was also increased. Control group showed bile ductules only in portal areas. Representative images from each group are shown in figure 3.
Bile ductules were analyzed by H&E staining. Control group: bile ductules (arrow) were found only in portal areas (PA); DEN 150 group: ductular proliferation (arrows) was observed within the hepatic parenchyma and also in PA; DEN 200 group: the number of bile ductules was increased (arrows) with a similar pattern as in DEN 150 group. Magnification: 400X.
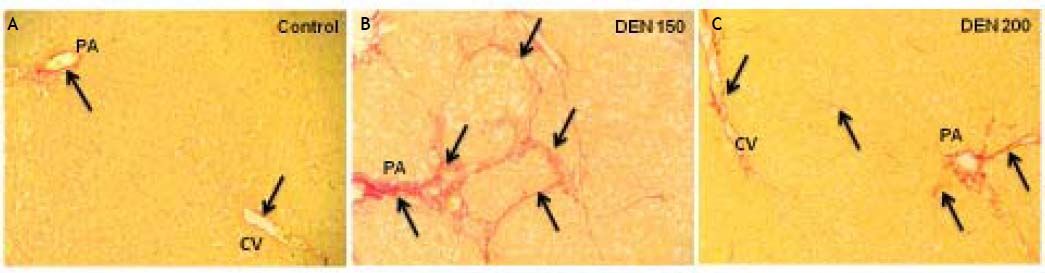
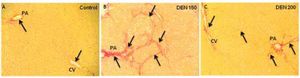
DEN 150 group presented the highest amount of total collagen (controls: 0.0032 ± 0.0004; DEN 150: 0.0068 ± 0.0008; DEN 200: 0.0041 ± 0.0005). Thin and abundant collagen fibers were increased in perisinusoidal spaces, portal areas and surrounding central veins. DEN 200 and control groups had a normal distribution and amount of collagen fibers. Representative images from each group are shown in figure 4. Quantification of the number of bile ductules and the amount of collagen fibers are summarized in figure 5.
Representative images of liver collagen deposition. Control group: thin collagen fibers (arrows) were seen especially in portal areas (PA) and surrounded central veins (CV); DEN 150 group: the amount of total collagen was evidently increased. Large collagen fibers (arrows) were distributed within the hepatic parenchyma; DEN 200 group: collagen deposition (arrows) was slightly increased compared to control group. Magnification: 200X.
Bile ductules proliferation and total collagen estimation. A. The amount of bile ductules was significantly higher in DEN 150 and DEN 200 groups than in control animals, n = 3. B. Total collagen was significantly higher in DEN 150 group than in control and DEN 200 groups, n = 3. Each bar represents the mean ± SE, *p ≤ 0.05
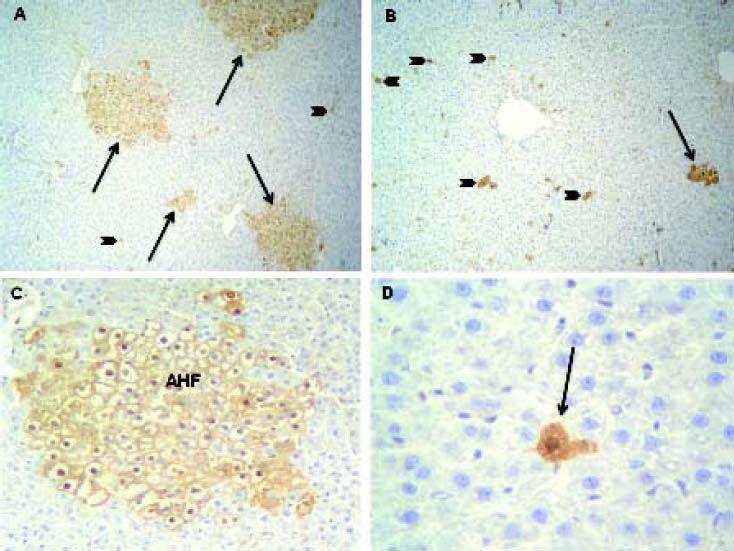
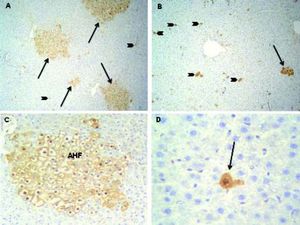
rGST-P has been described as the most effective single marker of hepatic preneoplasia in the rat.7 Consequently, immunohistochemical detection of this isozyme is the most widely used method for identification and quantitation of rat preneoplastic foci.13 Representative images of AHF from DEN 150 and DEN 200 groups are shown in figure 6.
Altered hepatic foci (AHF) immunostained with r-GST-P. A. DEN 150 group: r-GST-P positive foci (arrows) were distributed throughout the hepatic parenchyma. Few isolated initiated-hepatocytes (arrow heads) were also found. Magnification: 100X. B. DEN 200 group: r-GST-P positive AHF (arrows) were smaller than in DEN 150 group; furthermore, many isolated initiated-hepatocytes (arrow heads) were distributed throughout the liver parenchyma. Magnification: 100X. C. A representative image of an AHF from DEN 150 group. Magnification: 200X. D. A representative image of an initiated-cell from DEN 200 group (arrow). Magnification: 400X.
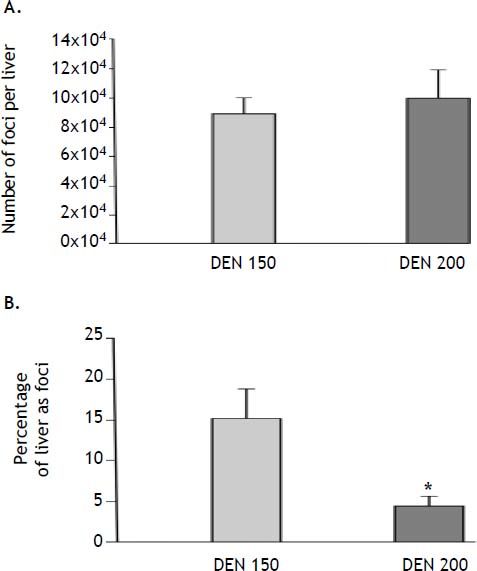
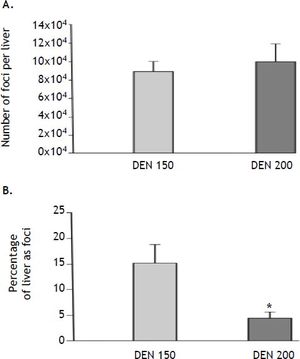
We analyzed the effect of treatments on the number and the volume of rGST-P-positive foci. Both parameters were determined because each of them measures different stages in the carcinogenic process. The number of AHF reflects the amount of initiated cells capable of developing into clones of AHF, whereas the volume percentage reflects the growth rate and total cellular population of the AHF in general.14 As expected, no foci were detected in control animals. On the other hand, animals subjected to DEN 150 and DEN 200 protocols did not show differences in the number of AHF per liver (DEN 150: 89,776 ± 9,734; DEN 200: 100,226 ± 19,873). However, animals from DEN 200 group showed a significant diminution (-70%) in the volume of liver occupied by foci (DEN 150: 15.2 ± 3.9 %; DEN 200: 4.5 ± 1.2 %). Results are summarized in figure 7.
Number and volume of altered hepatic foci (AHF) estimation. A. The number of AHF per liver did not show statistically significant differences between DEN 150 and DEN 200 groups, n = 4. B. The volume percentage of the liver occupied by foci was significantly lower in DEN 200 than in DEN 150 group, n = 4. Each bar represents the mean ± SE, * p ≤ 0.05
The natural history of liver cancer development involves several stages including initiation, promotion and progression. Initiation can be developed by the administration of a hepatic carcinogen at a necrogenic dose or by using a lower dose in combination with PH.15 We based our work on the one of Alvarez, et al.4 We reduced the frequency of chemical agents’ administration, and therefore, the total dose of carcinogens was diminished as well as animals’ manipulation. Besides, we compared both treatments to study early stage of hepatic preneoplasia in order to evaluate their effectiveness. To carry out our aims, we used a single dose of DEN (200 mg/kg body weight, intraperitoneally), and 2-AAF (20 mg/kg body weight) by gavage only two doses per week during three weeks, as we previously described. By using this new protocol which is simpler than the one of Alvarez, et al., rat livers developed AHFs. So, we can conclude that the experimental procedure presented in this work is a useful tool to obtain preneoplastic lesions in rat livers.
Transaminases had no statistical differences between groups whereas ChE values from DEN groups were higher than control group. Although ChE values fell within a normal range in all experimental groups, it would be expected lower values for ChE activity in DEN-treated groups than in controls. Nevertheless, increased ChE values may indicate that the ability of the liver to synthesize new proteins is restored after the carcinogen injury. ALP activity showed the lowest value in DEN 200 group. Previous unpublished results from our laboratory showed that animals from DEN 150 group presented cholestasis with ductular proliferation. Cholestasis increases ALP concentrations;16 however, DEN 150 group had no differences in ALP activity compared to control animals (Figure 2). Although cholestasis was not evaluated in the present study, we observed that rats from DEN-treated groups had the same increment in the number of bile ductules. So, we have to perform more studies to explain ALP results from DEN 200 group.
Percentage of PT is a good parameter to determinate hepatic vitamin K production.5 DEN 200 group had no statistical differences from control animals while DEN 150 group presented the lowest value of PT percentage among the experimental groups. In addition to serum parameters analysis, we found that DEN 200 group had no fibrosis. Meanwhile, DEN 150 group had higher total collagen amount compared to controls. These results let us to conclude that DEN 200 group was in better hemostatic and morphological conditions than DEN 150 group.
DEN-treated groups showed a significant increase in the number of bile ductules compared to control. Bile ductular cell hyperplasia has been shown to be a common response in a variety of clinical and experimental forms of liver injury.17 It is known that intrahepatic biliary epithelium cells, including those of the bile ductules and canals of Hering, by virtue of their apparent resistance to various hepatotoxic and hepatocarcinogenic environments, are equipped not only to survive, but may also be permitted to selectively proliferate under conditions that would be mitoinhibitory to normal hepatocytes.18
Analyzing the number and volume of AHF in the livers from treated groups we found that animals from DEN 150 and DEN 200 groups had not differences in the number of AHF per liver, and that animals from DEN 200 group showed a significant diminution in the volume of liver occupied by foci. The number of foci represents the number of initiated cells by DEN administration and foci size is in direct relation with the administration of 2-AAF.4 The number of AHF were equal in both treated groups; this means that DEN dose reduction in DEN 200 group did not affect AHF appearance but 2-AAF dose was insufficient to make the foci grow during treatment as much as in DEN 150 group. Nevertheless, DEN and 2-AAF doses in DEN 200 group were adequate to induce AHF in rat livers.
Considering all the results obtained we conclude that both protocols compared in this work are good alternatives to induce liver preneoplasia in rats and that the new proposed protocol is an effective and a simple methodology to provide a subclinic state of liver cancer.
Abbreviations- •
2-AAF: 2-acetylaminofluorene.
- •
AHF: altered hepatic foci.
- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
ChE: cholinesterase.
- •
DEN: diethylnitrosamine.
- •
PH: partial hepatectomy.
- •
PT: prothrombin time.
- •
rGST-P: placental form of rat Glutathione Stransferase.
This study was supported by the Secretary of Science, Technology and Innovation of Santa Fe, Scientific Project No 2010-093-11, Argentina.
AcknowledgementsThe authors acknowledge Histotechnologist Alejandra Inés Martinez for her excellent technical assistance and Wiener Laboratories SAIC (Rosario, Argentina) for providing the reagents for enzymatic determinations.