Large primary hepatocellular carcinoma (HCC) has a high mortality rate and a variety of treatments. Surgery and transcatheter arterial chemoembolization (TACE) are important treatments. Which could be better remain debatable. The objective of the study is to compare the long-term overall survival of surgical resection (SR) and the use of TACE in patients with large hepatocellular carcinoma.
Materials and MethodsWe assessed clinical trials through PubMed, Medline, Embase, and the Cochrane Library up to March 2022. Two researchers independently screened articles, extracted data, and assessed the study quality according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses)guidelines. The primary outcome was overall survival (OS). The secondary outcomes were OS after propensity scores matching (PSM) and progression-free survival (PFS).
ResultsA total of 14 studies, including 3609 patients, were enrolled in the meta-analysis. The meta-analysis indicated a significant improvement in the 1-year OS, 3-year OS, and 5-year OS favoring SR over TACE (OR = 2.19, 95% CI 1,60–3.00; OR = 3.47, 95% CI 2.47–4.88; OR = 2.72, 95% CI 2.03–3.64, p < 0.001, random model). The results were consistent across subgroups of tumor size and tumor numbers (p > 0.05). The pooled outcome indicated that 1-year OS, 3-year OS, and 5-year OS after PSM were higher in the SR group than in the TACE group (p < 0.001).
ConclusionsThis meta-analysis indicates that among patients with large primary hepatocellular carcinoma, the overall survival rate of patients undergoing surgical resection was higher than that of patients undergoing TACE.
Primary hepatocellular carcinoma (HCC) is a common malignant tumor of the digestive system. According to the latest statistical data, liver cancer ranks sixth in the incidence of malignant tumors and third in mortality [1]. East Asia and Africa have the highest incidence and mortality, while parts of Europe and the USA have an increasingly higher incidence [2,3]. As for larger HCC, liver resection is the preferred treatment according to the previous guideline [4–6]. And according to Barcelona Clinic Liver Cancer (BCLC) classification [7], HCCs beyond 5 cm still qualify as early-stage eligible for SR. However, the prerequisites for resection are based on liver function, portal hypertension, and the extent of hepatectomy and surgical invasiveness according to the EASL guideline [4]. The principle of TACE is to inject chemotherapeutic drugs and embolization agents into the supplying arteries of the tumor, resulting in ischemia and hypoxia of the tumor cells, thereby promoting apoptosis and necrosis [8]. TACE has been a significant non-surgical treatment for hepatocellular carcinoma in recent years [5,9,10], which has less trauma and definite curative effect so that some HCC patients who are not suitable for surgical resection can also get the chance of TACE [11].
In patients with large HCC (lesion> 5 cm), previous studies and guidelines have focused on HCC in the early stage of BCLC (single lesion>5 cm, or 2–3 nodules ≤3 cm), which recommends that SR should be the optimal treatment. TACE should be the treatment of choice for HCC patients with the BCLC B stage [12,13]. However, recent studies have indicated that in patients with multiple large HCCs, surgical resection has better overall survival than TACE [14–18]. A meta-analysis of 2619 patients also indicates that SR had better overall survival than TACE with intermediate HCC [19]. The previous analysis concentrating on solitary large hepatocellular carcinoma indicated that SR resulted in greater survivability and time to disease progression than TACE [20]. However, the number of included samples is small, and there is some heterogeneity in the paper. And the article focused on large solitary HCC. Recently, more studies have been published on SR and TACE in the treatment of large HCC, and most of them have conducted propensity-matching studies [14,15,18,21,22].
So, we do this meta-analysis to compare the survival of patients with primary large hepatocellular carcinoma treated with SR and TACE.
2Materials and Methods2.1Search strategyA search of the literature was conducted in PubMed, Medline, Embase, and the Cochrane Library up to May 2022. The search strategy included “hepatocellular carcinoma,” “hepatoma,” “liver cell carcinoma,” “hepatocarcinoma,” “HCC,” “liver cancer,” “large,” “huge,” “transarterial embolization,” “transarterial therapy,” “transcatheter arterial chemoembolization,” “TACE,” “hepatectomy,” “surgery resection,” “surgery,” “resection,” “survival” and “mortality.” Searches were all completed trials in human beings with abstracts or full texts. The supplemental Table 1 presented an example of the search strategy.
2.2Inclusion and exclusion criteriaTwo researchers read the literature review independently of each other, and the arguments were solved by consensus. The following criteria were included to ensure the reliability of the research: (1) adults patients (> 18 years old) with primary hepatocellular carcinoma; (2) tumor size ≥ 5 cm; (3) the treatments of HCC have included both TACE and SR; (4) articles of which the original data could be extracted; (5) articles published as full-text in any language. The study must meet all inclusion criteria. Studies were excluded if they met any of the following characteristics: (1) patients with diffuse hepatic carcinoma or metastasis in other organs; (2) overlapping or duplicate reports; (3) nonhuman experiments; (4) tumor size < 5 cm; (5) absence of basic data or primary outcomes.
2.3Data extractionThe following data were extracted: publication characteristics, study regions, patient characteristics, the sample size of patients, treatment of HCC, diameter of the tumor, and endpoints. The primary endpoints of the studies were overall survival (OS), including 1-year OS, 3-year OS, and 5-year OS. The secondary outcomes were overall survival after propensity scores matching (PSM) and progression-free survival (PFS).
2.4Study quality assessmentIncluded studies were subjected to a risk of bias assessment by using the ROBINS-I (a tool for assessing the quality of non-randomized studies) [23]. The quality of evidence for outcomes was examined using the grading of recommendations assessment, development, and evaluation (GRADE) approach [24].
2.5Subgroup analysesWe performed two subgroup analyses, including tumor size (larger than 5 cm, larger than 10 cm) and tumor numbers (single tumor, multiple tumors).
2.6Trial sequential analysisWe performed the sequential trial analysis of the study. We calculated the information size required to demonstrate or reject a 20% relative benefit increment (surgical treatment being the outcome of benefit). We assumed a 50% event proportion in the control group, which was roughly the median and average control group event proportion. We used a type I error of 5% and a type II error of 20%.
2.7Statistical analysisAll data were analyzed using Review Manager (version 5.3) or State 14.0. The number of surviving patients at each period (1, 3, and 5 years) was treated as a dichotomous variable with the number of survivors and total numbers of patients extracted from the included studies. Between-study heterogeneity was explored by Cochrane's Q and I2 tests. A fixed effect model was used in the absence of significant heterogeneity (I2 < 50%), or the random effect model was used. We conducted sensitivity analyses by State. The possibility of study effects was assessed qualitatively by the visual estimate of the funnel plot. We regarded two-sided probability values of < 0.05 as statistically significant.
2.8Ethical statementThis systematic review and meta-analysis were conducted using the predefined protocol and following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. The review protocol was registered in PROSPERO (CRD 42021244307).
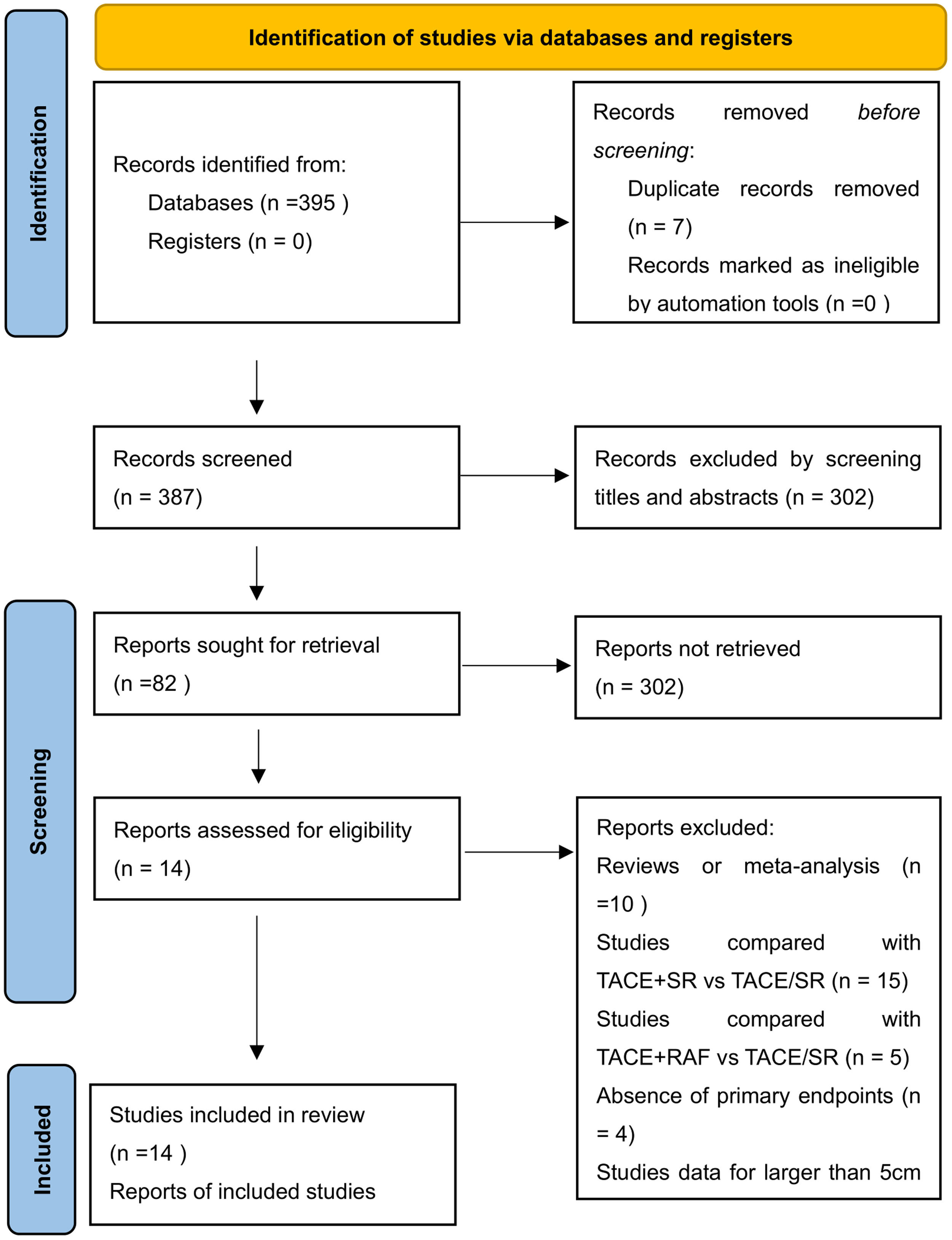
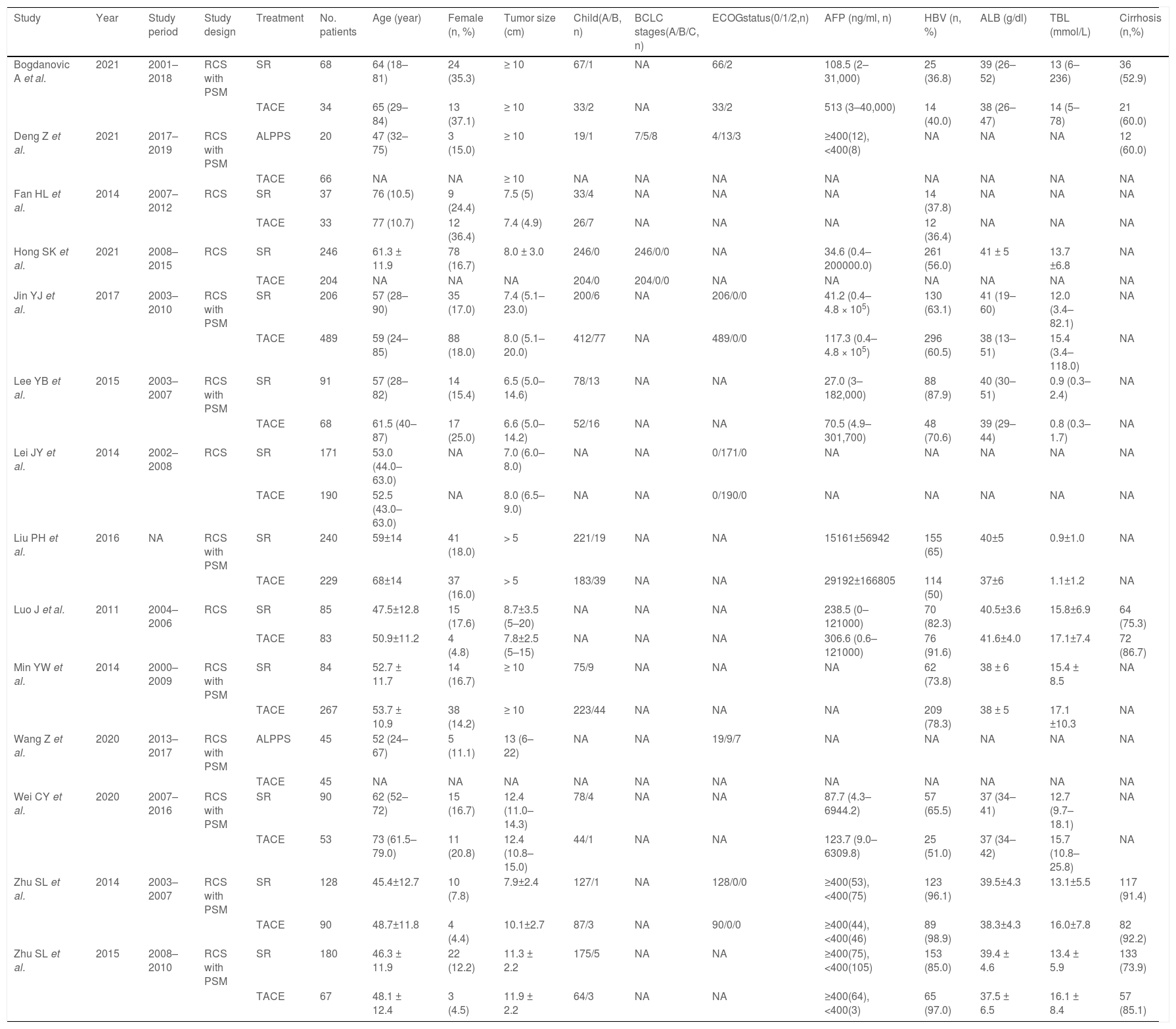
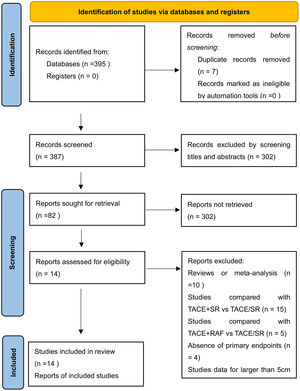
3Results3.1Literature search and basic characteristicsInitially, a total of 395 references were included in the primary search in the major databases. After deleting duplications, 387 pieces of research were selected. By screening titles and abstracts, 82 studies were included, and finally, 14 studies were enrolled in our meta-analysis according to the inclusion criteria [14–18,21,22,25–31]. A diagram of the study selection is shown in Fig. 1. A total of 3609 patients were enrolled in the meta-analysis, and the basic characteristics of included studies are listed in Table 1. Most of the studies came from Asian countries, while only 1 study came from a European country [14]. The 1-year overall survival rate was reported in 11 studies [15–18,22,25–30], while 13 studies reported a 3-year overall survival [14–18,22,25–31]. And 11 studies reported long-term overall survival for 5 years [14,16,17,21,22,25–30]. In addition, there were 10 studies that reported overall survival after PSM [14,15,17,18,22,25–29], and four studies reported PFS [14,15,21,25].
The basic characteristics of enrolled studies.
RCS Retrospective cohort study; PSM propensity score matching; SR surgery resection; TACE transarterial chemoembolization; ALPPS association liver partition and portal vein ligation for staged hepatectomy. NA Not available.
The risk of bias was shown in supplemental Table 2, 5 trials had a low risk of bias, and nine trials had a moderate risk of bias. Using the GRADE summary of the evidence, the quality of evidence for the primary outcome was low (supplemental eTable 3).
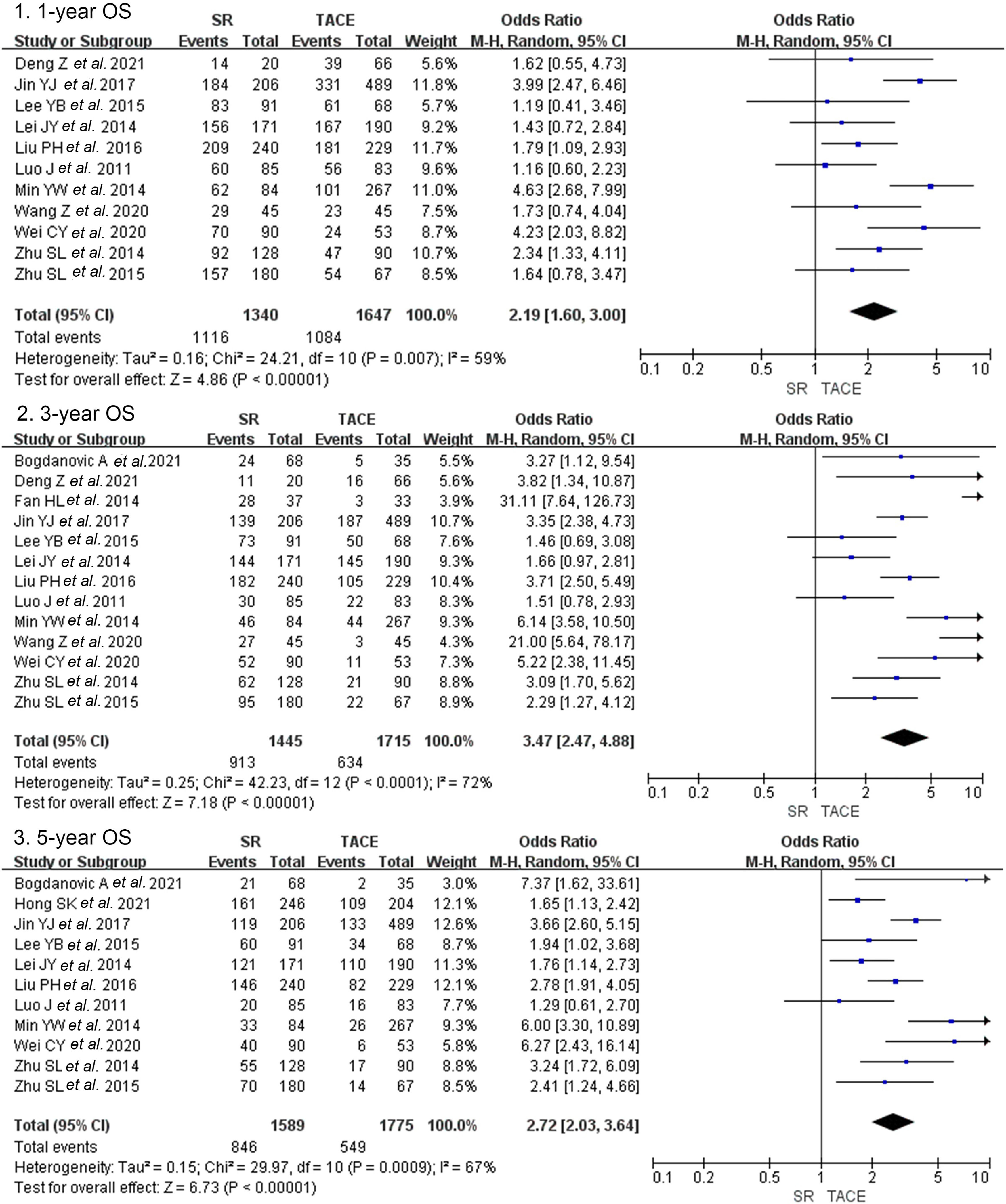
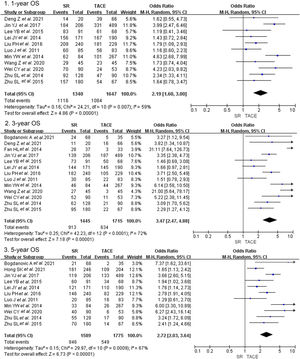
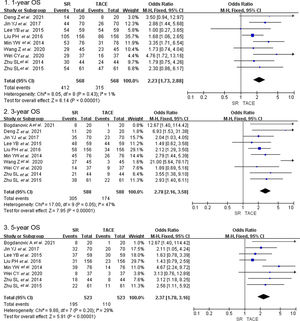
3.2Primary outcomes3.2.1Overall survivalCrude overall survival in the SR group and TACE group was 83.3% vs. 65.8, 63.2% vs. 40.0%, and 53.2% vs. 30.9% in 1-year, 3-year, and 5-year. The combined analysis of 11 cohorts covering 2987 patients described the treatment between the SR group and TACE group [13–16,20,23–28]. And the pooled outcome indicated that SR was superior to TACE (OR = 2.19, 95% CI 1,60–3.00, p < 0.001, random model, Fig. 2) on 1-year OS. A total of 3160 patients included in 13 studies indicated that the three-year overall survival rate was higher in the SR group than in the TACE group (OR = 3.47, 95% CI 2.47–4.88, p < 0.001, random model). The same result was presented at 5-year overall survival (OR = 2.72, 95% CI 2.03–3.64, p < 0.001, random model).
Forest plot of overall survival of studies compared SR with TACE in patients with large HCC. Each square denotes the OR for each trial compared with the corresponding 95% confidence intervals. The size of the square is directly proportional to the amount of information contributed by the trial. The diamonds represent the overall outcome of OR and 95%CI.
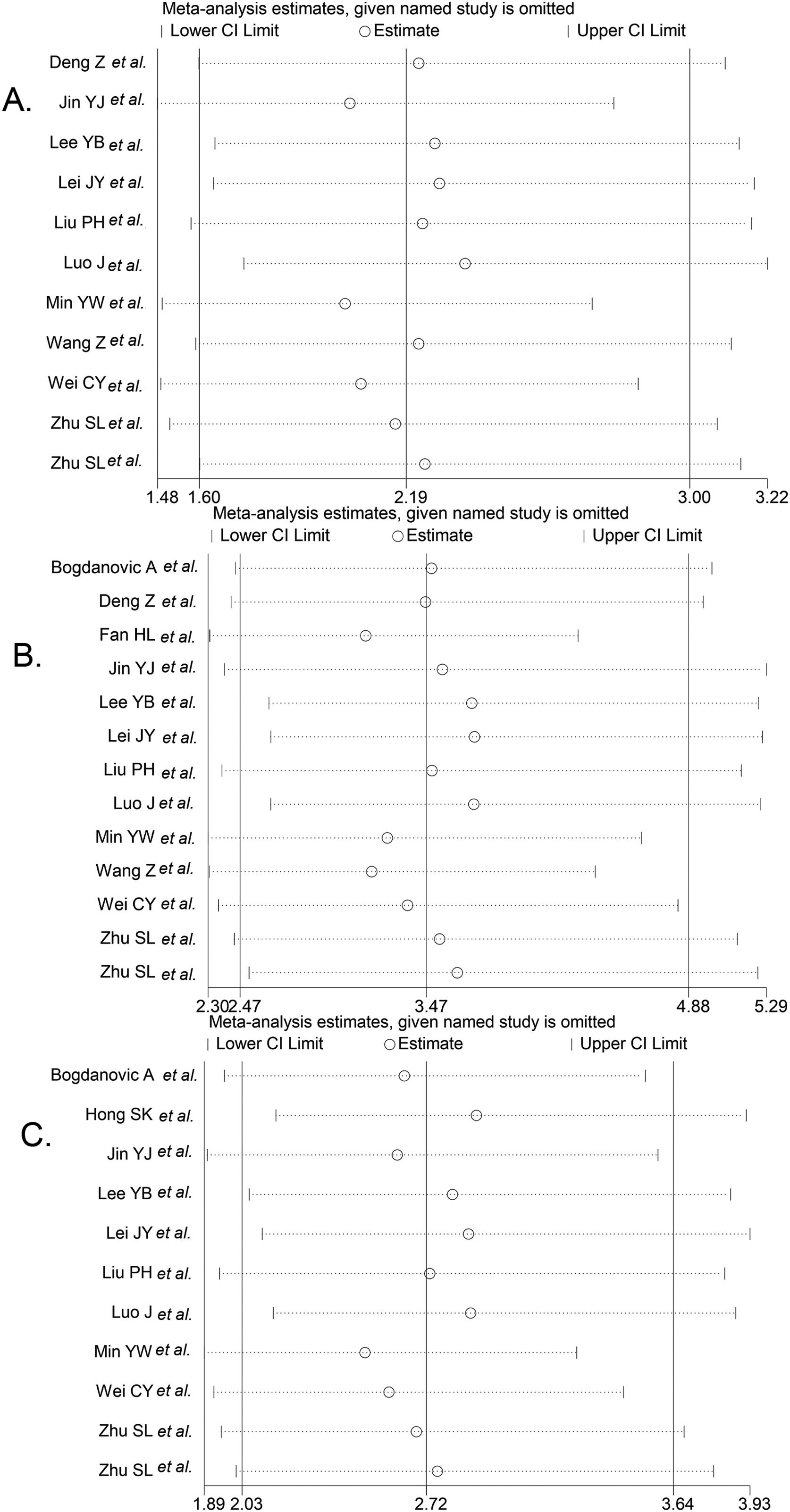
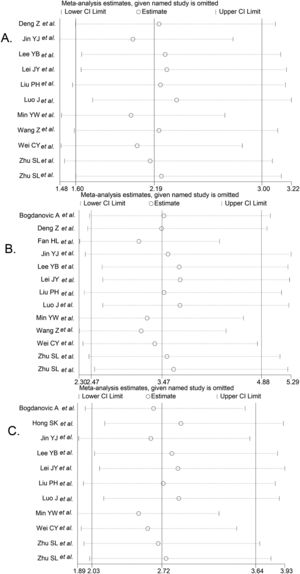
We performed the sequential trial analysis of the study. We calculated the required information size to 3280 patients. As the number of patients included in the meta-analysis exceeded the required information size, we applied the results that were acceptable (supplemental Fig. 1). Funnel plot analysis showed no asymmetry (supplemental Fig. 2). Sensitivity analysis suggested that significant changes in OR values were produced by the exclusion of any study (Fig. 3).
Result of sensitivity analysis. The middle vertical line indicates the combined OR, and the two vertical lines represent the 95% CI values. Every hollow round indicates the pooled OR when the left study was omitted in a meta-analysis with a random model. Fig. 5A shows the sensitivity analysis of 1-year OS, Fig. 5B shows the sensitivity analysis of 3-year OS, and Fig. 5C shows the sensitivity analysis of 5-year OS.
Due to the heterogeneity of the results, subgroup analyses were performed. 1-year OS and 3-year OS in the SR group were higher than in the TACE group both in tumor size > 10 cm group and tumor size 5–10 cm group, and no significant differences were found between the two groups (p > 0.05, supplemental Fig. 3). However, the 5-year overall survival rate of HCC patients with tumors larger than 10 cm suggested that SR was more effective than TACE and there was a significant difference (p = 0.02). The same results were found in the subgroup analysis of tumor numbers. The results indicated that 1-year OS, 3- year OS, and 5-year OS in the SR group were higher than in the TACE group both in the single lesion group and the multiple lesions group, and no significant differences were found between the two groups (p > 0.05, supplemental Fig. 4).
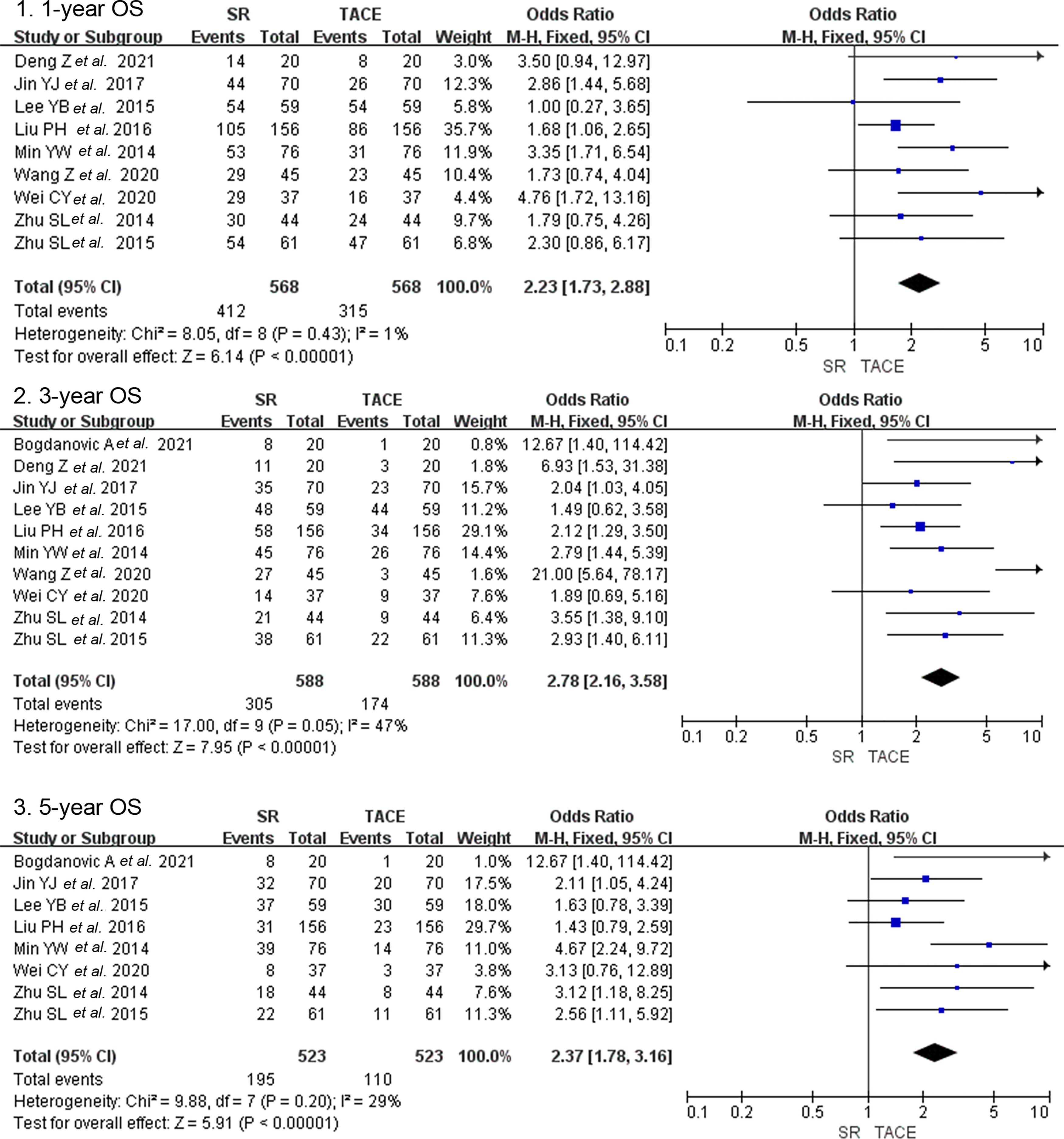
3.3Secondary outcomes3.3.1Overall survival after PSMThere were ten studies that included 1176 patients who reported overall survival after PSM [14,15,17,18,22,25–29]. One-year overall survival in the SR group and the TACE group was 72.5% and 55.5% after PSM. And the OS after PSM was 51.9% vs. 29.6% and 37.3% vs. 21.0% in 3-year and 5-year follow-ups between groups. Nine studies with a combined 1136 patients provided data on the 1-year OS after PSM and found significantly higher survival of the SR group in subjects with the TACE group (OR = 2.23, 95% CI 1.73–2.88, p < 0.001, fixed model, Fig. 4). Ten studies that enrolled 1176 patients indicated that three-year overall survival after PSM was higher in the SR group than in the TACE group (OR = 2.78, 95% CI 2.16–3.58, p < 0.001, fixed model). The same result was presented in a total of 8 studies that included 1046 patients on 5-year OS after PSM (OR = 2.37, 95% CI 1.78–3.16, p < 0.001, fixed model).
Forest plot of overall survival after propensity score matching of studies compared SR with TACE in patients with large HCC. Each square denotes the OR for each trial compared with the corresponding 95% confidence intervals. The size of the square is directly proportional to the amount of information contributed by the trial. The diamonds represent the overall outcome of OR and 95%CI.
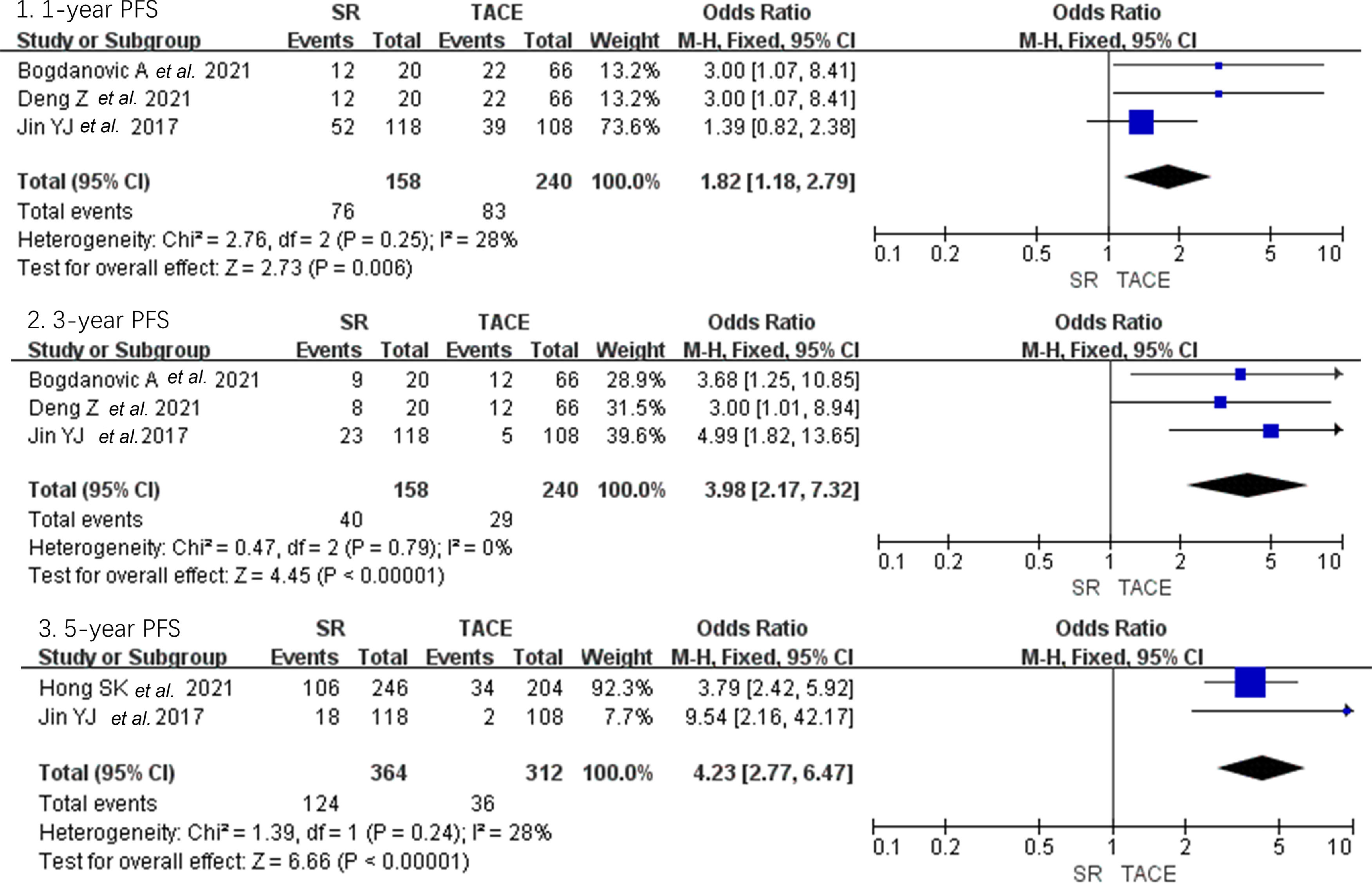
As shown in Fig. 5, only four studies reported the PFS of large HCC and found significantly higher PFS of the SR group in subjects with TACE group in 1-year, 3-year, and 5-year (OR = 1.82, 95% CI 1.18–2.79; OR = 3.98, 95% CI 2.17–7.32; OR = 4.32, 95% CI 2.77–6.47, p < 0.01, fixed model) [14,15,21,25].
Forest plot of progression-free survival of studies compared SR with TACE in patients with large HCC. Each square denotes the OR for each trial compared with the corresponding 95% confidence intervals. The size of the square is directly proportional to the amount of information contributed by the trial. The diamonds represent the overall outcome of OR and 95%CI.
This systemic review meta-analysis compared the effectiveness of SR and TACE based on 14 studies that included 3609 patients and demonstrated that SR is associated with improved overall survival when compared with TACE for patients with large HCC. The results were consistent after PSM analysis. Subgroup analysis suggests that SR is superior to TACE regardless of tumor size and tumor numbers. In patients with large HCC, the SR group shows better PFS than the TACE group.
Previous guidelines did not have a significant classification of large HCC. According to the BCLC algorithm, solitary large HCC > 5 cm was defined as stage A, and hepatic resection should be the optimal treatment [7]. As for BCLC stage B, EASL and AASLD guidelines recommended TACE as the treatment of choice [4,5]. The role of SR for large HCC remains controversial. According to previous guidelines, SR is not indicated for BCLC B stage HCC patients because of poor overall survival. On the other hand, strict surgical guidelines preclude most patients from surgical treatment. The previous selection criteria for SR presented in the previous (EASL)/(EORTC) Clinical Practice Guidelines in 2012 were solitary tumors and very well-preserved liver function, hepatic vein to portal system gradient lower than 10 mmHg or platelet count ≥100,000/ml [32]. However, many studies have shown that in selected patients with intermediate-stage BCLC HCC, SR can give better survival than TACE [16,30,33]. A meta-analysis of 9 studies with 2619 patients indicated that SR had better overall survival than TACE for patients with intermediate-stage HCC [19]. Our meta-analysis focused on large HCC, which is not limited to the BCLC stage, and indicated that SR was superior to TACE. The crude 1-year, 3- year, and 5-year overall survival rate in the SR group and TACE group was 83.3% vs. 65.8, 63.2% vs. 40.0%, and 53.2% vs. 30.9%. The previous study shows that the early stage of HCC may achieve 70% 5-year survival rates [7]. Our research included large HCC combined early stage and intermediate stage, and the overall survival was lower than it. Stevens et al. included four studies on large HCC and found that cumulative overall survival rates in the SR group and TACE at 1, 3, and 5 years were 85% vs. 75%, 72% vs. 54%, and 61% vs. 36%, which was consistent with our results.
Besides, most studies included were retrospective cohort studies with PSM. Our results demonstrated that after PSM, in patients with large HCC, SR got better overall survival than TACE. Hyun et al. also compared the survival of 5,986 patients after SR and TACE; after PSM, both BCLC stage B and BCLC stage C patients showed significantly better OS for SR compared to TACE [34]. Both tumor size and tumor number influence operation. A previous study showed that from tumor sizes larger than 5 cm, the complete necrosis rate was all below 20%, which might weaken adhesiveness within the tumor, thus facilitating the invasion of HCC [35]. The overall survival of HCC larger than 10 cm treated by SR at five years ranged from 30.8% to 44.4%, according to previous studies [14,22]. Our analysis indicated that SR was the better treatment in both groups, which was consistent with previous studies [20,36]. Some studies indicated that pre-TACE could be beneficial for large HCCs while others got controversial results [9,10].
In patients with large liver cancer, due to the large mass, relatively complex tumor supplying arteries, and often accompanied by vascular invasion and portal vein tumor thrombus, TACE tumor necrosis rate was low and postoperative tumor angiogenesis was likely to lead to recurrence and metastasis, whereas the long-term prognosis was not ideal. A recent meta-analysis by Zhang et al. suggested that Resection might be a meaningful choice for hepatocellular carcinoma with portal vein thrombosis [37].
The majority of HCC in Asian countries is attributable to viral hepatitis, especially chronic HBV infection. In recent years, chronic HCV also contributed to HCC. In our meta-analysis, the percent of HBV-positive ranged from 36.8% to 98.9%, while the percent of cirrhosis ranged from 52.9% to 92.2%. The EASL guideline did not get a consensus on "criteria" for SR in cirrhosis, and the decision of SR should be influenced by liver function assessment, portal hypertension, and the extent of hepatectomy and surgical invasiveness [4]. The previous review suggested that in HCC with liver cirrhosis, SR was superior to TACE in 1-year and 3-year OS [38]. Previous studies showed that different surgical approaches affected the prognosis [39]. Liu et al. and Jabir MA et al. indicated that the anterior approach resulted in better operative and survival outcomes compared with the conventional approach, which was the preferred technique for major right hepatic resection for large HCC [40,41]. Deng et al. and Wang et al. showed that long-term survival after ALPPS was significantly better than that after TACE [15,18].
Other non-surgical may also gain an important role in the treatment of HCC; transarterial radioembolization (TARE) is increasingly used as an alternative to TACE for the treatment of HCC. A recent meta-analysis indicated that TARE provided significantly longer TTP than TACE but did not significantly differ in terms of OS [42]. Some studies also indicated that it was an important downstaging therapy for unresectable hepatocellular carcinoma prior to hepatic resection [43,44]. One study concentrated on the large HCC and found that in treating patients with BCLC-B2 substage HCC, TARE treatment could be a better choice than TACE, especially in those with a large tumor [45]. However, there was no comprehensive assessment of the effect of TARE vs. TACE vs. surgical resection, which may need further research.
Certainly, there are still some limitations underlying this meta-analysis. First of all, the enrolled studies are retrospective cohort researches, which relatively influence the accuracy of the study, and most studies have a bias in the selection of participants into the study, which may influence the results. However, PSM analysis partially attenuated the effects. Second, the number of surviving patients at each period is treated as a dichotomous variable with the number of survivors and total numbers of patients extracted from the included studies; OR value is used for analysis, and there is moderate heterogeneity in this article. However, PSM analysis showed no significant heterogeneity, which confirmed the conclusion. Third, most of the included studies are from Asian countries, lacking relevant studies from other countries, and the scope of the included studies is not wide enough. Fourth, there were only 2 or 3 related studies in partial subgroup analysis, which may affect the accuracy of the research results. Then, there was no definite classification of BCLC stages in most of the included studies, and the articles with classification included BCLC A/BCLC B stages. Different stages have a great impact on the prognosis of the disease. Finally, most of the studies did not mention if there were any follow treatments after primary therapy, which may have an effect on overall survival. Besides, TSA analysis is conducted in this study to meet the sample size required for the conclusion. And PSM analysis improves the quality of the articles included in the study.
5ConclusionsThis meta-analysis indicates that among patients with large primary hepatocellular carcinoma, the overall survival rate of patients undergoing surgical resection was higher than that of patients undergoing TACE. Further, more rigorous prospective controlled studies are needed to validate the results.
Author contributionsLuke Zhou: visualization, conceptualization, data curation, writing – original draft. Mao Zhang: data curation, formal analysis, supervision. Siyu Chen: visualization, conceptualization, formal analysis, supervision.
FundingThe study is funded by the Deyang Municipal Commission of Health and Family Planning (2018SZS078).
Data availability statementThe data supporting this study's findings are available from references (including studies).
We thank all authors whose publications could be adopted in our meta-analysis.