Patients with hepatocellular carcinoma (HCC), the fifth most common cancer worldwide, display a highly variable clinical course, suggesting that HCC encompasses several biologically distinct subtypes. This heterogeneity has the potential to impede both treatment decisions and prognostic predictions for patients with HCC. One distinct, albeit rare, subtype of HCC is combined hepatocellular-cholangiocarcinoma (cHCC-CC), which overall carries a poorer prognosis than HCC and cholangiocarcinoma (CC) alone. This review discusses predominantly the histopathologic and pathogenetic intricacies of this tumor and highlights the need for an accurate diagnosis of this specific HCC subtype.
Combined hepatocellular-cholangiocarcinoma (cHCC-CC) is a rare malignant primary hepatic tumor defined as one that contains unequivocal intimately mixed elements of both hepatocellular carcinoma (HCC) and cholangiocarcinoma (CC).1 Although the World Health Organization (WHO) now recognizes cHCC-CC as a distinct subtype of hepatic malignancy, the diagnosis, prognosis and treatment of this neoplasm remain ill-defined.2 Furthermore, the subclassification of this already uncommon tumor into a) classical type and b) stem-cell associated type raises the potential for further diagnostic uncertainty within the hepatology community. This review aims predominantly to discuss the unique histologic features of cHCC-CC and to raise awareness of this significant, albeit rare, HCC subtype amongst clinicians and pathologists alike.
EpidemiologyBased on a study of over 20,000 patients with primary hepatic cancer captured within SEER (Surveillance, Epidemiology, and End Results Program of the National Cancer Institute) cHCC-CC had an overall incidence of 1.3%; the age and sex specific incidence and the geographical distribution are similar to those for HCC.2–4 It is important to note that the reported and true incidence may differ, given the significant potential for misdiagnosis of cases with inadequate tissue sampling of either component on needle core biopsies.
PathogenesisThe pathogenesis of cHCC-CC remains uncertain. Its frequent association with hepatitis B and hepatitis C virus suggests that it may be closely related to pure hepatocellular carcinoma (HCC);5,6 however, a large study carried out in the United States of America showed that the incidence of positive hepatitis B or C serology and cirrhosis is less than 15% in cHCC-CC, which is similar to that of cholangiocarcinoma (CC).7 There is evidence that hepatic stem/progenitor cells may play an important etiological role. One theory is that cHCC-CC arises from ‘intermediate’ cells resem- bling stem/progenitor cells, which show divergent differentiation.6,8,9
Early genetic studies determining the allelic status of chromosome arms in both the hepatocellular and cholangiolar components of cHCC-CC have provided evidence to suggest that a proportion of these tumors derive from a single clone, since both components within the same tumor share identical allelic losses.9,10 This would support the theory that the cell of origin of these tumors may be an ‘intermediate cell’ that then undergoes divergent differentiation. Some cases however, show a biclonal nature and do not share any allelic losses. These tumors likely represent ‘collision tumors’ rather than true cHCC-CCs, whereby two separate tumors develop in close proximity to one another and do not show evidence of intimate admixture or foci of transition.9,10
Macroscopic Appearance and Clinical PresentationMacroscopically cHCC-CCs are not significantly different from ‘conventional’ HCCs. Most tumors are unifocal and appear paler than the adjacent uninvolved hepatic parenchyma. Depending on the amount of the HCC component, the tumor may show greenish discoloration due to bile accumulation; hemorrhage and necrosis are common in larger lesions. Often ‘snakelike’ masses of tumor involve the portal vein and, less frequently, the hepatic veins.
Tumors with a large CC component tend to be more firm and fibrotic due to the fibrous stromal component usually present in CC.2
Accurate preoperative non-invasive diagnosis of cHCC-CC is extremely difficult with conventional radiologic techniques despite intensive analysis of these tumors.11–14 Many are misdiagnosed as HCC or CC depending on which component predominates.11,12 Correct preoperative diagnosis is desirable however, because the frequency of lymph node metastasis in cHCC-CC has been reported to be as high as 70%, a frequency comparable to that of CC, making lymph node dissection a necessity if curative resection is to be attempted.11
Alpha-fetoprotein (AFP) levels have, not surprisingly, been found to be raised in cHCC-CC; however, a significant difference in AFP levels between cHCC-CC and HCC has not been demonstrated.6,11 The presence of a raised AFP along with findings suggestive of a CC on imaging, or in combination with an elevated CA19-9, should however alert clinical investigators to the possibility of a cHCC-CC.11,13
The Role for BiopsyBiopsy should be performed for preoperative diagnosis with a needle core biopsy being the preferred method of obtaining tissue. This is because, unlike a fine needle aspiration, a core biopsy allows the pathologist not only to diagnose malignancy, but also to evaluate the architecture/pattern of the tumor, which is imperative for accurate diagnosis of cHCC-CC. The limitation of needle core biopsy is that, due to the inherent heterogeneity of these tumors, the material biopsied may only contain one of the two components, resulting in misdiagnosis as HCC or CC.
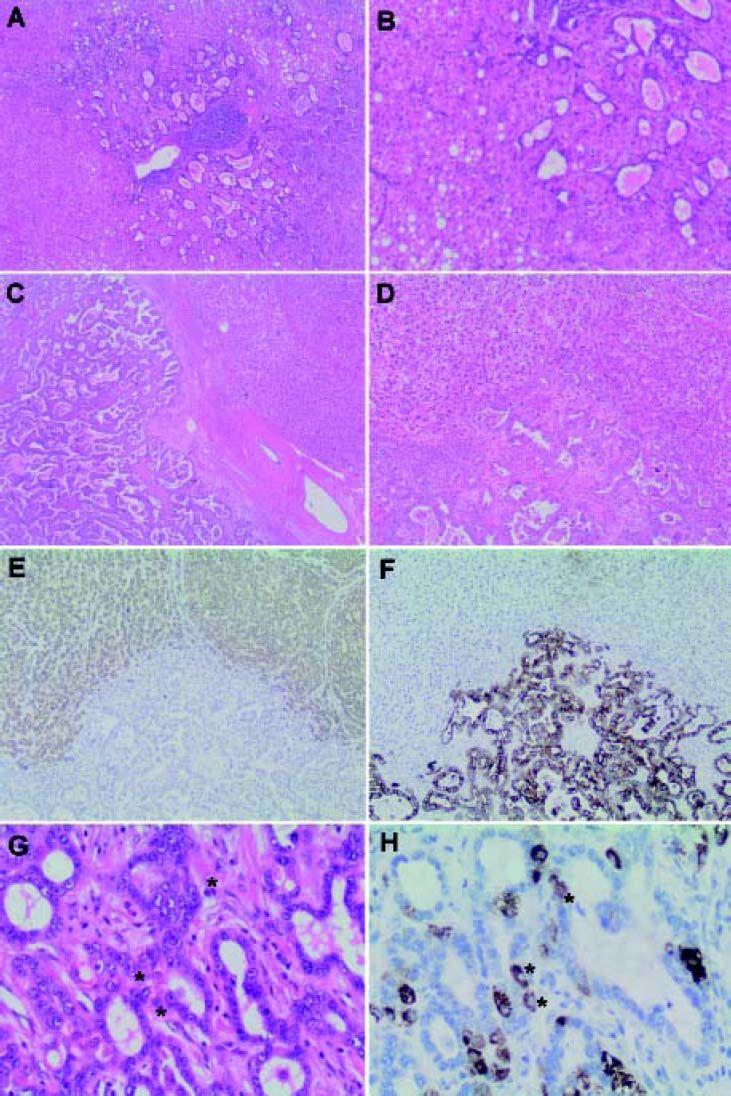
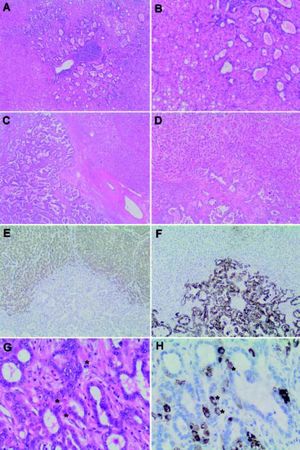
Microscopic Appearance and Immunohistochemical FeaturesThe WHO defines the hepatocellular component of cHCC-CC by the presence of bile production, bile canaliculi and a trabecular pattern of growth. The glandular component is defined by the presence of true gland formation with mucin production. In order to be classified as a ‘true’ cHCC-CC both elements of the tumor should be intimately mixed and show evidence of transition zones which are comprised of cells with intermediate morphology (Figure 1).2 The presence of these transition zones is important in order to distinguish cHCC-CC from ‘collision tumors’ (where both tumor components are clearly separated or are situated side by side and do not show any areas of transition).1
Combined hepatocellular-cholangiocarcinoma (cHCC-CC). A. Classical type, usually shows a malignant hepatocellular component with distinctive intermixed foci of cholangiocarcinoma (B). ‘Collision’ tumors (C) show separate hepatocellular (HCC) and cholangiocarcinoma (CC) components that lie in close proximity to each other but do not intermix (D). HepPar1 (E), a specific hepatocyte marker, stains the malignant hepatocellular component, while cytokeratin 7 (CK 7), a marker of biliary differentiation, stains the malignant cholangiolar component (F) highlighting the lack of intermingling of these elements. In contrast, transition zones (G) in classic cHCC-CC show cells with mixed ‘intermediate’ morphology (*). These ‘ntermediate’ cells (H) share morphological and immunohistochemical properties with both hepatocytes and cholangiocytes as highlighted by HepPar1, which stains positively in some of the tumor cells comprising the cholangiolar component (*). Magnification: A x 20; B x400; C-F x 100, G and H x 400.
Current WHO guidelines classify cHCC-CC into two subtypes:
- •
cHCC-CC classical type and
- •
cHCC-CC with stem cell features.
The latter of which is extremely rare and is further subcategorized into the following three subtypes:2
- •
Typical subtype.
- •
Intermediate cell subtype, and
- •
Cholangiocellular subtype.
The most common form of cHCC-CC is the classical type. It contains areas of typical looking HCC, intermixed with CC and identifiable transition zones, where the two components merge and show tumor cells with intermediate morphology (Figure1). The hepatocellular component may be well, moderately or poorly differentiated. The cholangiolar com- ponent is usually a typical adenocarcinoma (cholangiocarcinoma), which may also be well, moderately or poorly differentiated. The CC component is typically accompanied by the distinctive abundant fibrotic stroma characteristic of cholangiocarcinoma.
Immunohistochemistry (IHC) plays an important role in confirming the separate components of the tumor as well as demonstrating transition zones. Confirmation of a hepatocellular carcinomatous element is provided by immunohistochemical staining with HepPar-1 (hepatocyte paraffin 1 monoclonal antibody; granular cytoplasmic staining), canalicular staining with polyclonal CEA (carcinoembryonic antigen) or CD10 and demonstration of sinusoidal “capillarization” by CD34. Alpha-fetoprotein (AFP) may or may not be positive and, as such, is of limited utility. Bile may be also produced and can be highlighted by histochemical staining in difficult scenarios.2,15
The cholangiocarcinomatous component shows positive immunohistochemical staining for CK7, CK19 and MOC31. It is important to note that positive staining of tumor cells with these immunostains must be interpreted in combination with the morphologic features of the tumor as, although positivity with these stains are consistent with biliary differentiation, they are not specific for it alone, and may be positive in hepatocellular components also.2,15,16 A mucin stain (i.e. periodic acid-Schiff stain after diastase digestion (dPAS) or mucicarmine) can also be very helpful in demonstrating the cholangiolar adenocarcinomatous (cholangiocarcinomatous) component by highlighting intracytoplasmic mucin.2
Transition zones have been shown to stain for both typical biliary cytokeratins CK7 and CK19, as well as the hepatocellular marker HepPar-1 (Figure 1). Interestingly, they have also been shown to concurrently display positive signals for in situ hybridization for albumin mRNA, a sensitive and specific hepatocellular marker that has not been reported in cholangiocarcinoma. This positivity for both hepatocellular (albumin mRNA ISH) and biliary (cytokeratin IHC) markers confirm the microscopic impression of biphenotypic differentiation in these tumors.15 Transition zones may also contain cells with immunophenotypical features of stem/progenitor cells as demonstrated by cKIT positivity; which is of importance in classifying cHCC-CC with stem cell features.17
Combined Hepatocellular-Cholangiocarci Noma with Stem Cell FeaturesIn the liver, several cell types have the longevity required to be the cell of origin of a cancer: hepatocytes, cholangiocytes and progenitor cells. The latter are located in the most peripheral branches of the biliary tree, the ductules and canals of Hering.18 If these stem cells predominate in a cHCC-CC, the tumor should be designated as a “combined hepatocellular-cholangiocarcinoma with stem cell features” of which there are three separate subtypes (typical, intermediate cell and cholangiocellular), which can be differentiated by morphologic and immunophenotypic characteristics.19 Although these subtypes can be pathologically defined, they are not yet considered as distinctive clinical entities, as it is to date not apparent whether significant biological differences between them exist.2
The first subtype, the typical one, is characterized by nests of mature appearing hepatocytes with peripheral clusters of small cells with hyperchromatic nuclei and a high nuclear to cytoplasmic ratio. These clusters of cells stain positively with CK7, CK19, NCAM1/CD56 (nuclear cell adhesion molecule), cKIT and/or epithelial cell adhesion molecule (Ep-CAM) and hence recapitulate the morphological and immunohistochemical features of stem/progenitor cells.19
The intermediate cell subtype consists of tumor cells with intermediate features between hepatocytes and cholangiocytes. Morphologically these tumor cells are small and oval shaped with hyperchromatic nuclei and scant cytoplasm arranged in either trabeculae, solid nests or strands, and set within a desmoplastic stroma. Poorly defined gland like structures may be present but well formed glands are not seen. There is no significant cellular atypia and there is no evidence of mucin production. The tumor cells show typical cytoplasmic expression of HepPar-1 and simultaneous expression of CK19 or CEA; cKIT expression is also common.16
Lastly, the cholangiolocellular subtype has small cells with a high nuclear to cytoplasmic ratio and hyperchromatic oval shaped nuclei which are arranged in a tubular, cord like, anastomosing pattern also referred to as an ‘antler-like’ pattern, morphologically mimicking cholangioles, embedded in a dense fibrous stroma. Cellular atypia is mild and there is no evidence of mucin production. Immunohistochemically the tumor cells are positive for CK19, cKIT, NCAM1/CD56 and EpCAM. Previously this tumor was classified as a special subtype of cholangiocarcinoma but is now considered to be a subtype of cHCC-CC with stem cell features.8
Prognosis and Management of Combined HepatocellularcholangiocarcinomaMany studies that have looked at prognosis in cHCC-CC in comparison to HCC and CC have differed in their outcomes, likely due to the rarity of this tumor and the resultant small sample sizes, and differences in classification criteria over time. Nevertheless, the overall trend has shown that cHCC-CC has a significantly poorer outcome than HCC and CC even after attempted curative resection. Likelihood of survival is dependent on disease stage at the time of diagnosis.11,20,21 Prognosis and tumor recurrence after liver transplant for patients with cHCC-CC has been shown to be significantly worse than for patients that undergo transplantation for HCC.22 There are, as yet, no data to determine the prognosis of cHCC-CC classical type in comparison to cHCC-CC with stem-cell features. Nevertheless, it is important to attempt to identify and report these subtypes, as numerous studies from multiple other solid tumors, including HCC and pancreatic ductal adenocarcinoma, have shown that the presence of stem-cell features correlates with more aggressive tumor behavior, including tumor recurrence, chemoresistance and metastases even after complete resection.23
Complete surgical resection of the tumor, provided liver function will be adequately preserved post operatively, currently remains the only chance of cure and is the treatment of choice for non-cirrhotic patients with localized disease; however, recurrence risk is reportedly higher in these patients than in those with HCC or CC.24 Since cHCC-CC tends to have hepatic and portal venous infiltration similar to HCC and lymph node metastases similar to CC, hepatic resection with lymph node dissection is the current recommended treatment for non-cirrhotic patients.11,24
A small number of case reports have described these tumors as having a good response to transarterial chemoembolization (TACE) and sorafenib, yet these treatments require further evaluation.24,25
SummaryTrue cHCC-CC shows macroscopic, radiological and clinical overlap with HCC and CC making accurate preoperative diagnosis exceptionally difficult. Preoperative radiological diagnosis is accurate in only a minority of cases. Morphological and immunophenotypical overlap with HCC and CC can also make histological diagnosis challenging. Serum AFP, although raised in cHCC-CC, is not specific to this entity and may also be raised in HCC and other non malignant hepatic conditions including cirrhosis; however, an increase in both serum AFP and CA19-9 may be helpful in raising suspicion of cHCC-CC. Core needle biopsy is preferable to fine needle aspiration as the method of obtaining tissue for diagnosis; however, the inherent heterogeneity of these tumors may lead to an incorrect diagnosis of HCC or CC. Histological intermingling of both HCC and CC elements are required in order to be classified as a true cHCC-CC rather than a “collision tumor”, whereby both elements lie in close proximity to each other but do not mix. cHCC-CC should also show areas of distinct morphologic transition whereby the tumor cells show biphenoty-pic differentiation with unique morphological and immunophenotypical properties that resemble stem/ progenitor cells. The presence of these stem/progenitor cells is thought to be the reason why these tumors exhibit such aggressive biological behaviour and poor prognosis with a reported 36% 5 year survival.21 In addition, common genetic features between both hepatocellular and cholangiolar components of this tumor have been demonstrated, lending further credence to the theory that cHCC-CC tumors arise from a single clone with divergent differentiation, and therefore truly represent a different entity to “collision tumors”, which do not share genetic characteristics.
Attempts to recognise and identify the stem cell subtypes of these tumors is important to allow further study of their biological significance, as this may have implications for future therapeutic intervention. Due to the rarity of these tumors, in particular cHCC-CC with stem cell features, prognostic evidence is limited; however, the data available to date suggest that overall these are aggressive tumors, with surgical resection, where feasible, the only chance of cure and stage of tumor at diagnosis being the most important factor determining survival.11,24,25 In addition to resection of the primary tumor, lymph node dissection is also recommended due to the fact that cHCC-CC can behave similarly to CC, with a high propensity for lymph node metastasis, especially if this component predominates.11 Other treatment options including TACE and systemic chemotherapy require further evaluation.24,25
In conclusion, cHCC-CC clearly represents a distinct subtype of hepatic carcinoma, which appears to display aggressive biological behaviour and poor prognosis.21 Preoperative clinical diagnosis is currently exceptionally difficult. Greater awareness of cHCC-CC as a distinct entity, understanding of the pathogenesis, and further investigation of potential novel and targeted therapies for cHCC-CC is required in order to improve the outcome for patients with this tumor.