Background & Aim. Transarterial chemoembolization (TACE) or sorafenib is recommended for hepatocellular carcinoma BCLC stages B and C respectively. We studied the role of combination of TACE and sorafenib in BCLC stages B/C.
Material and methods. We undertook an observational study on a cohort of cirrhotics with HCC from August 2010 through October 2014. Patients in BCLC stages B/C who had received TACE and/or sorafenib were included. mRECIST criteria were used to assess tumor response. The primary end point was overall survival.
Results. Out of 124 patients, 47.6% were in BCLC-B and 52.4% in BCLC-C. Baseline characteristics were comparable. The predominant etiology was cryptogenic (37.2% and 38.5%, p = NS). 49.1% in BCLC-B and 56.9% in BCLC-C had received TACE+sorafenib. In BCLC-B, the overall survival improved from 9 months (95% CI 6.3-11.7) using TACE only to 16 months (95% CI 12.9-19.1) using TACE+sorafenib (p < 0.05). In BCLC-C, addition of TACE to sorafenib improved the overall survival from 4 months (95%CI 3-5) to 9 months (95%CI 6.8-11.2) (p < 0.0001). As per mRECIST criteria, patients on TACE+sorafenib had reduced progressive disease (37.8% vs. 83.3%), improved partial response (43.2% vs. 3.3%) and one had complete response compared to those on sorafenib alone (p < 0.0001) in BCLC-C but not in BCLC-B group. Hand foot syndrome was noted in 27.7% patients on sorafenib and post TACE syndrome in 80.2% patients, but both were reversible. No major adverse events were noted.
Conclusion. TACE+sorafenib was more effective than TACE or sorafenib alone in HCC BCLC stages B or C with a significant survival benefit and improved tumour regression especially in BCLC-C patients.
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm and the third most frequent cause of cancer death.1 HCC is the leading cause of death in compensated cirrhotics.2 Tumor characteristics in form of size, number of lesions and vascular invasion and host characteristics in form of Child's status of liver disease and overall performance status of the patient dictates the therapeutic options and its outcome.3 While radiofrequency ablation, curative resection and liver transplantation are the optimal treatment modalities for Barcelona Clinic Liver Cancer (BCLC) Stage 0 and A, palliation is the only option for BCLC stage B and stage C.4 Curative resection in BCLC stage B and C is hampered by anatomical constraints of the intrahepatic tumor and portal hypertension, as reflected by the Child's score or the Model for End-stage Liver Disease (MELD) score.
The recommended palliation for BCLC stage B and stage C is transarterial chemo embolization (TACE) and sorafenib respectively.5 TACE has also been used as a neo-adjuvant procedure to downstage a tumour before liver transplantation6 or as an adjuvant for tumour recurrence after resection.7 Sorafenib, an oral multikinase inhibitor is the only available chemotherapeutic drug with a survival benefit in advanced HCC.8 TACE induced hypoxia can lead to release of angiogenic cytokines from tumour cells which can enhance tumour recurrence and metastasis.9 Sorafenib exerts an anti-angiogenic effect by blocking the vascular endothelial growth factor receptor-2 and -3 and platelet-derived growth factor receptor tyrosine kinase.10 Hence combination of TACE and sorafenib has shown to be an effective strategy with an improved time to tumor progression and overall survival in advanced HCC.11 However, the recently published multi-centric STORM trial in early HCC had found that addition of sorafenib after resection or ablation had no additional benefit in recurrence free survival compared to placebo.12
The aim of our study was to evaluate whether a combination of TACE and sorafenib can improve the outcome in BCLC stage B and C patients.
Material and MethodsCirrhotic patients with hepatocellular carcinoma belonging to BCLC stage B and C, presenting to the Institute of Liver Diseases and Transplantation, Global Health City, India from August 2010 to October 2014 were included in this retrospective study. Diagnosis of HCC was based on classical findings of arterial enhancement with contrast and an “early wash out” during imaging on contrast enhanced computed tomography triple phase or contrast enhanced magnetic resonance imaging triple phase.13 BCLC staging classification14 was used to stratify the patients and identify those in stages B and C. The exclusion criteria were patients who have received previous treatments for hepatocellular carcinoma such as radiofrequency ablation or percutaneous ethanol injection, Child's C status, total bilirubin more than 3 mg/dL,15 significant cardio-pulmonary co-morbidity, presence of any other illness that significantly affects survival, not willing for TACE or sorafenib, lack of minimum three months follow up except for non-survivors. Patients with alcoholic liver disease were advised strict alcohol abstinence and those with cirrhosis due to viral etiology were on antiviral therapy.
The study protocol conformed to the Declaration of Helsinki of 1975, as revised in 1983 and was approved by the Institutional Ethics Committee. The study followed the STROBE guidelines for reporting of observational studies. Informed consent was obtained from each patient prior to the interventional procedure.
TreatmentUntil mid 2012, conventional TACE using doxorubicin with lipiodal was the procedure of choice for BCLC stage B. Repeat TACE was done in those showing partial tumor response on follow up imaging. Segmental thrombosis of the portal vein (left or right), regional lymph nodes involvement such as periportal or paraaortic were not considered as an absolute contraindication for TACE. After the introduction of sorafenib in 2013, patients in BCLC stage B group who received TACE were also started on sorafenib if they were willing for the same. In BCLC stage C group, the patients were started on sorafenib as per the BCLC recommendations.14 However, those patients who were not in exclusion criteria for TACE were offered the option of TACE and if willing, received TACE as well and continued on sorafenib in the BCLC stage C group. Soraf-enib in a dose of 200 mg twice a day was introduced 5 days after TACE when angiogenesis was expected. If the dose was tolerated, it was further increased to 400 mg twice a day and continued. Sorafenib was discontinued if there were untoward adverse effects, progression of liver disease to Child Pugh Class C or lost to follow up. External application of Epsom salt (magnesium sulphate) was recommended in both hands and feet to prevent Hand Foot Syndrome.16 Complications pertaining to TACE and sor-afenib were noted.
Follow-upModified Response Evaluation Criteria In Solid Tumors (mRECIST criteria)17 was used to monitor progression/regression of tumour on imaging at three monthly intervals in the first year and every six months thereafter for next two years.18
- •
Complete response. Disappearance of any intratu-moral arterial enhancement in all target lesions
- •
Partial response. A minimum of 30% reduction in tumor size (sum of all diameters of enhancing lesions) from baseline
- •
Stable disease. Any lesions that did not not qualify for partial response or progressive disease
- •
Progressive disease. A minimum of 20% increase in tumor size (sum of all diameters of enhancing lesions taking as reference; the sum of the smallest sum of the diameters of target lesions recorded since treatment was started).17,18
The primary end-point was overall survival. For survival analysis, a minimum follow up of 3 months was considered mandatory, except for non survivors. Last date of follow up was obtained from either outpatient attendance or by telephonic interview. The secondary end points were tumor response defined as per mRECIST criteria and treatment related adverse events.
Statistical analysisIt was a time bound study from August 2010 to October 2014. All consecutive cirrhotics with hepatocellular carcinoma fulfilling the inclusion and exclusion criteria were included in the study.
Baseline parametric data was expressed as the proportion, mean ± standard deviation and median with range. The differences in the groups were analyzed using chi-square test, unpaired t-test or Mann-Whitney test as appropriate. Paired t-test was used to compare the change before and after the intervention. The cumulative probability of overall survival was determined by Kaplan-Meier plots and compared by log-rank test. The p value < 0.05 was considered statistically significant. All statistical tests were performed using SPSS for Windows version 20 (SPSS Inc., Chicago, IL).
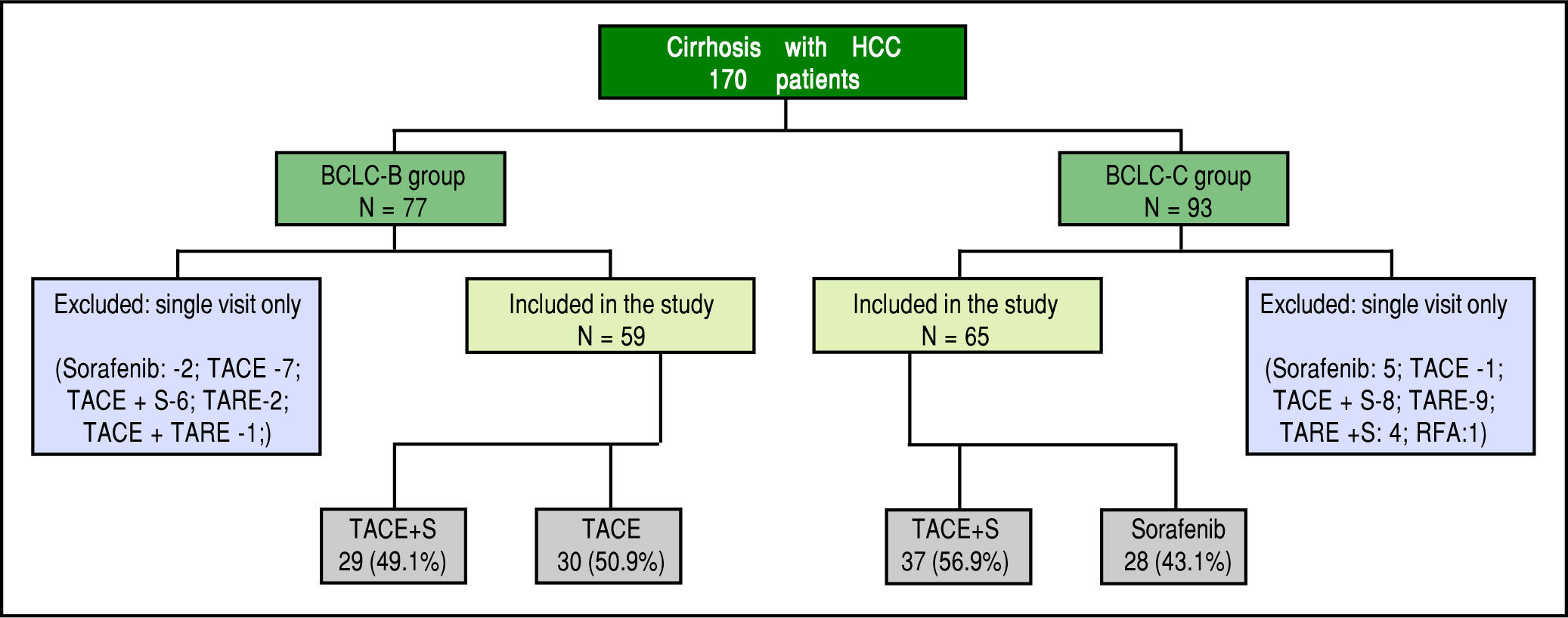
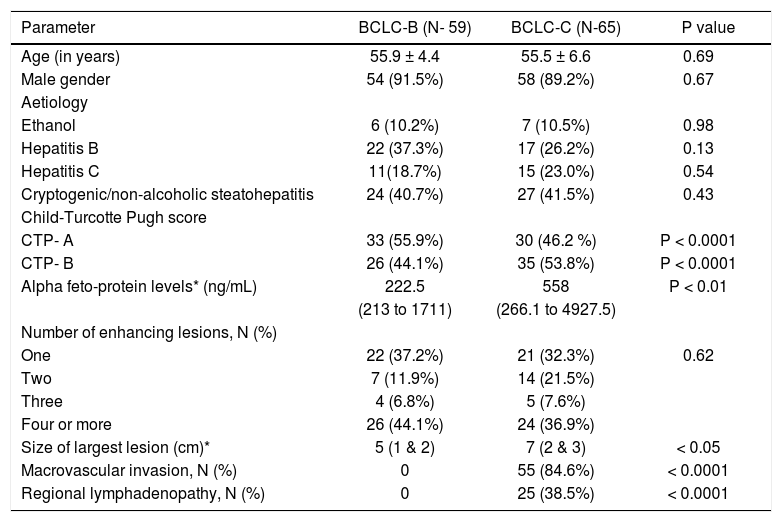
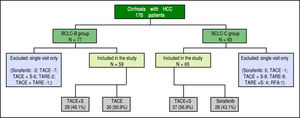
ResultsA total of 170 cirrhotics with hepatocellular carcinoma were seen from August 2010 to October 2014. After careful assessment, 124 patients who fulfilled the inclusion and exclusion criteria were included in the study (Figure 1). 59 (47.6%) patients were in BCLC-B and 65 (52.4%) were in BCLC-C. Majority were male gender (91.1%). The two study groups were well matched with respect to demographic characteristics, clinical and laboratory data (Table 1).
Baseline characteristics of the study cohort.
| Parameter | BCLC-B (N- 59) | BCLC-C (N-65) | P value |
|---|---|---|---|
| Age (in years) | 55.9 ± 4.4 | 55.5 ± 6.6 | 0.69 |
| Male gender | 54 (91.5%) | 58 (89.2%) | 0.67 |
| Aetiology | |||
| Ethanol | 6 (10.2%) | 7 (10.5%) | 0.98 |
| Hepatitis B | 22 (37.3%) | 17 (26.2%) | 0.13 |
| Hepatitis C | 11(18.7%) | 15 (23.0%) | 0.54 |
| Cryptogenic/non-alcoholic steatohepatitis | 24 (40.7%) | 27 (41.5%) | 0.43 |
| Child-Turcotte Pugh score | |||
| CTP- A | 33 (55.9%) | 30 (46.2 %) | P < 0.0001 |
| CTP- B | 26 (44.1%) | 35 (53.8%) | P < 0.0001 |
| Alpha feto-protein levels* (ng/mL) | 222.5 | 558 | P < 0.01 |
| (213 to 1711) | (266.1 to 4927.5) | ||
| Number of enhancing lesions, N (%) | |||
| One | 22 (37.2%) | 21 (32.3%) | 0.62 |
| Two | 7 (11.9%) | 14 (21.5%) | |
| Three | 4 (6.8%) | 5 (7.6%) | |
| Four or more | 26 (44.1%) | 24 (36.9%) | |
| Size of largest lesion (cm)* | 5 (1 & 2) | 7 (2 & 3) | < 0.05 |
| Macrovascular invasion, N (%) | 0 | 55 (84.6%) | < 0.0001 |
| Regional lymphadenopathy, N (%) | 0 | 25 (38.5%) | < 0.0001 |
The predominant aetiology of cirrhosis was non-alcoholic steatohepatitis / cryptogenic in both groups (37.2% and 38.5%, p = NS). These patients were labelled as cryp-togenic after ruling out other etiologies by doing viral se-rologies, ceruloplasmin, ANA profile, immunoglobulin G, iron studies and ferritin. As shown in Table 1, there were higher proportion of patients in Child's A status compared to Child's B in BCLC-B group (55.9% vs. 46.2%) and vice versa in BCLC-C group (44.1% vs. 53.8%) respectively (p < 0.0001). 29 (49.1%) patients in BCLC-B group and 37(56.9%) patients in BCLC-C group had received combination of TACE and sorafenib (Figure 1). The median follow up was slightly higher in BCLC-B group at 7 months (1.7 - 33) compared to 6 months (2-26) in BCLC-C group (p < 0.05). The median duration of treatment in BCLC- B patients was 6.1 months (range 2-33) in TACE alone group and 8 months (range 1.7-29) in TACE+ sorafenib group. In BCLC stage C group, it was 4 months (range 2-12) in sorafenib alone group and 7 months (2.5-26) in TACE + sorafenib group.
Tumour characteristicsThe average size of the largest tumour nodule was 5 cm (1-12) in BCLC-B group compared to 7 cm (1-16) in BCLC-C group (p < 0.05). We also analysed for any difference in the tumor characteristics in the two treatment groups categorized under Child's A and B status in each BCLC stage (Tables 2 and 3). There was no difference in tumor size between the two treatment groups in Child's A unlike those in Child's B patients, wherein the tumors were slightly smaller in the combination treatment group. None of the patients in BCLC-B group had mac-rovascular, loco-regional or distant metastasis. However, in BCLC- C group, macrovascular invasion was present in 55(84.6%) patients and regional lymphadenopathy in 25 (38.5%) (p < 0.001) (Table 1). When we compared the portal vein involvement in BCLC- C between the two treatment groups in Child's A and B patients, there was no difference noted between the two treatment groups (Table 3).
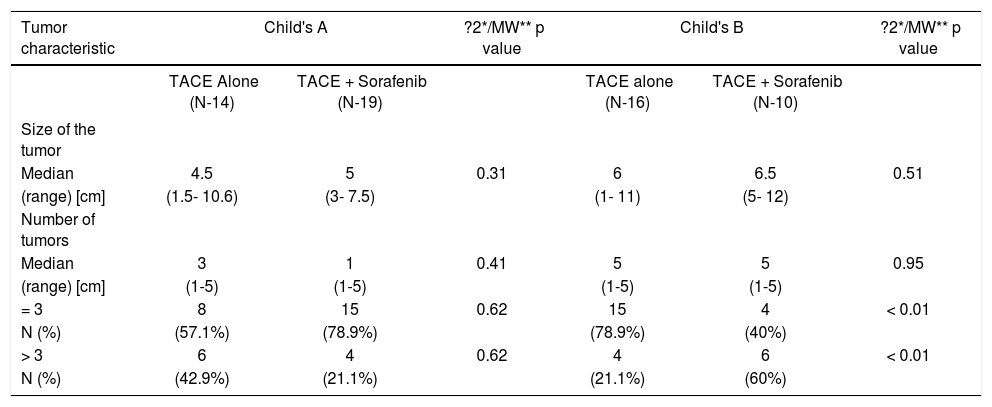
Comparison of tumor characteristics between the two treatment groups in Child's A and B in BCLC stage B.
| Tumor characteristic | Child's A | ?2*/MW** p value | Child's B | ?2*/MW** p value | ||
|---|---|---|---|---|---|---|
| TACE Alone (N-14) | TACE + Sorafenib (N-19) | TACE alone (N-16) | TACE + Sorafenib (N-10) | |||
| Size of the tumor | ||||||
| Median | 4.5 | 5 | 0.31 | 6 | 6.5 | 0.51 |
| (range) [cm] | (1.5- 10.6) | (3- 7.5) | (1- 11) | (5- 12) | ||
| Number of tumors | ||||||
| Median | 3 | 1 | 0.41 | 5 | 5 | 0.95 |
| (range) [cm] | (1-5) | (1-5) | (1-5) | (1-5) | ||
| = 3 | 8 | 15 | 0.62 | 15 | 4 | < 0.01 |
| N (%) | (57.1%) | (78.9%) | (78.9%) | (40%) | ||
| > 3 | 6 | 4 | 0.62 | 4 | 6 | < 0.01 |
| N (%) | (42.9%) | (21.1%) | (21.1%) | (60%) | ||
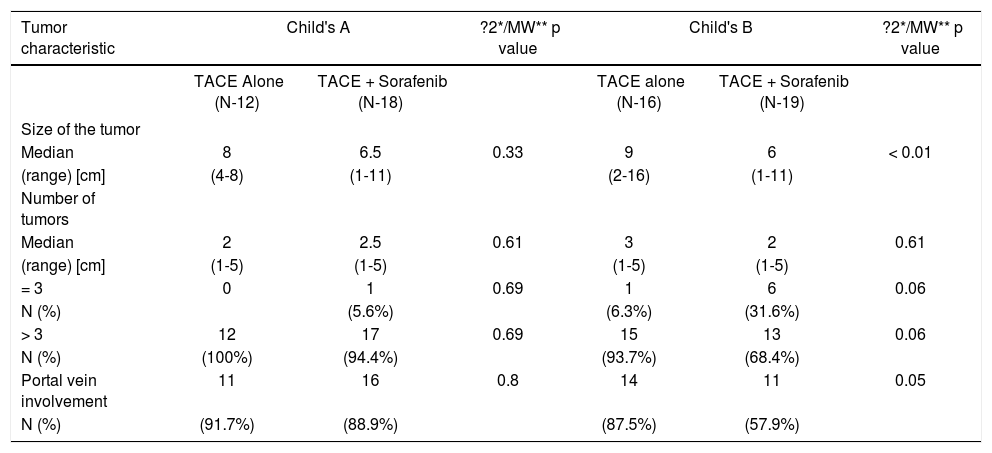
Comparison of tumor characteristics between the two treatment groups in Child's A and B in BCLC stage C.
| Tumor characteristic | Child's A | ?2*/MW** p value | Child's B | ?2*/MW** p value | ||
|---|---|---|---|---|---|---|
| TACE Alone (N-12) | TACE + Sorafenib (N-18) | TACE alone (N-16) | TACE + Sorafenib (N-19) | |||
| Size of the tumor | ||||||
| Median | 8 | 6.5 | 0.33 | 9 | 6 | < 0.01 |
| (range) [cm] | (4-8) | (1-11) | (2-16) | (1-11) | ||
| Number of tumors | ||||||
| Median | 2 | 2.5 | 0.61 | 3 | 2 | 0.61 |
| (range) [cm] | (1-5) | (1-5) | (1-5) | (1-5) | ||
| = 3 | 0 | 1 | 0.69 | 1 | 6 | 0.06 |
| N (%) | (5.6%) | (6.3%) | (31.6%) | |||
| > 3 | 12 | 17 | 0.69 | 15 | 13 | 0.06 |
| N (%) | (100%) | (94.4%) | (93.7%) | (68.4%) | ||
| Portal vein involvement | 11 | 16 | 0.8 | 14 | 11 | 0.05 |
| N (%) | (91.7%) | (88.9%) | (87.5%) | (57.9%) | ||
- •
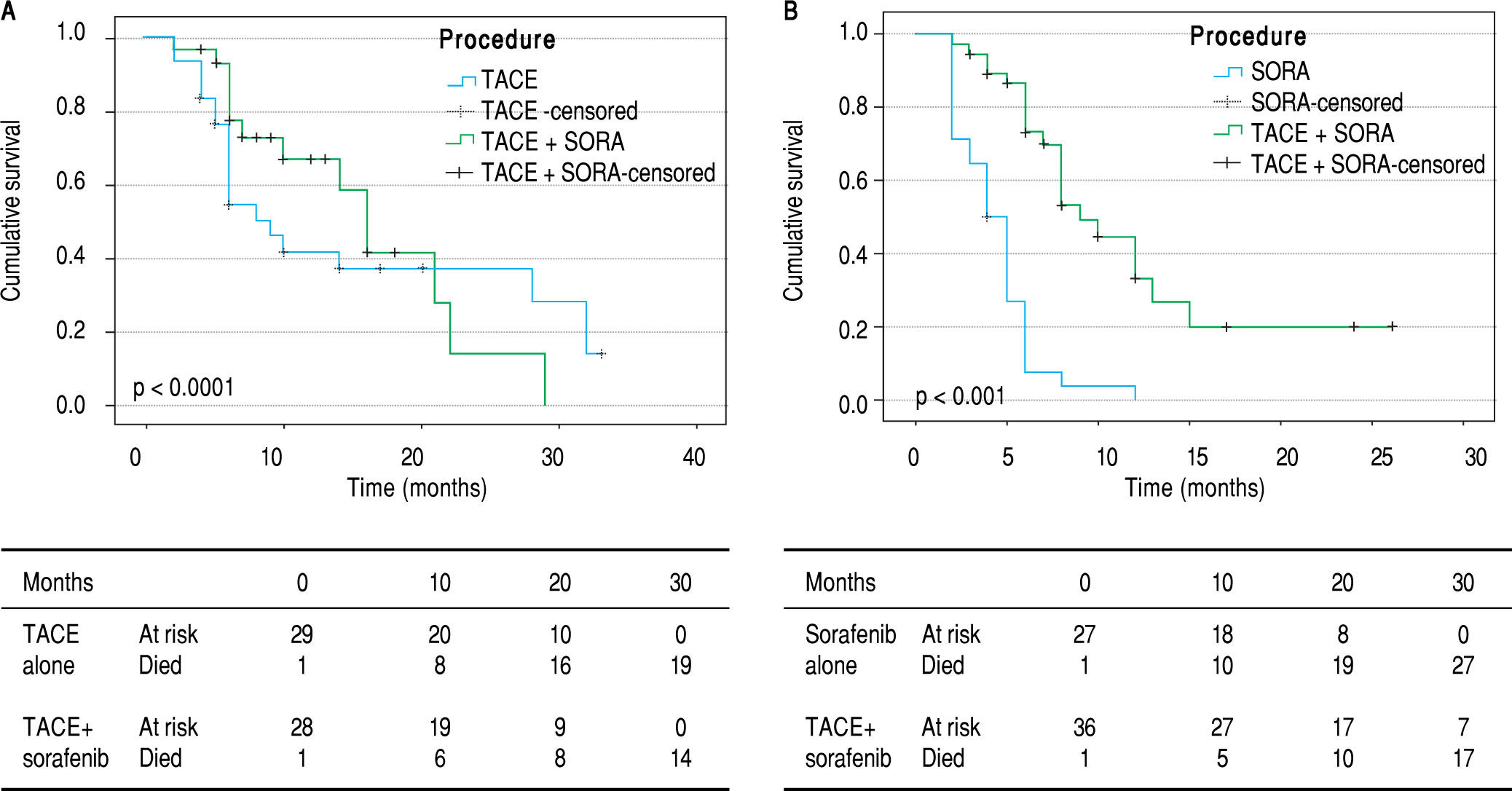
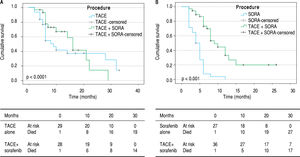
Overall survival. In BCLC-B group, the overall cu mulative probability of survival increased from 9 months (95% CI 6.3-11.7) using TACE only to 16 months (95% CI 12.9-19.1) using TACE and sorafenib (p < 0.05). In BCLC-C group, addition of TACE to sorafenib improved the overall survival from 4 months (95% CI 3-5) to 9 months (95% CI 6.8-11.2) (p < 0.0001) (Figure 2).
- •
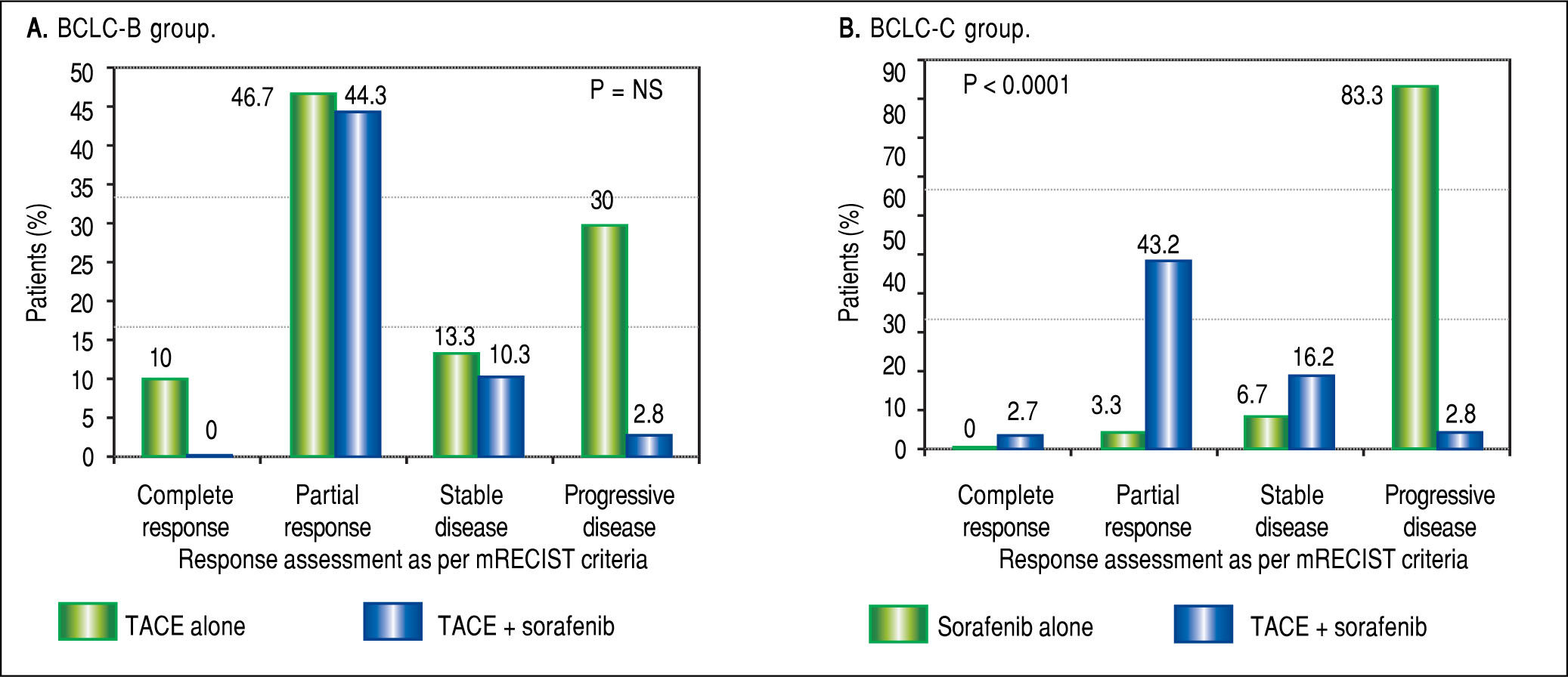
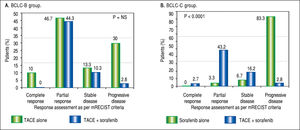
Tumor response to TACE with or without soraf-enib. Based on mRECIST criteria, combination of TACE and sorafenib had reduced incidence of progressive disease (37.8% vs. 83.3%), improved partial response (43.2% vs. 3.3%) and one patient had complete response compared to those on sorafenib alone (p < 0.0001) in BCLC-C group. In BCLC-B group, addition of sorafenib to TACE did not lead to any significant difference in tumor reponse noted between the two groups (Figure 3).
- •
Complications. Sorafenib related complications such as hand foot syndrome were present in 26 patients (27.7%) and TACE related complications (Post TACE syndrome) in 77 patients (80%). Post TACE syndrome included fever, right upper quadrant discomfort, nausea and vomiting, loss of appetite and liver function test showing transaminitis. However, all patients with post TACE syndrome had received hydration and symptomatic treatment with improvement in next two to three days. No major adverse events were observed in either group.
Management of hepatocellular carcinoma requires a multidisciplinary approach involving the hepatologist, in-terventional radiologist and liver surgeon. The major prognostic factors in HCC patients are related to tumor characteristics namely number and size of tumor nodules, presence of vascular invasion and extra-hepatic invasion, host factors such as degree of liver function defined by Child-Pugh's class and general health status defined by Eastern Co-operative Oncology Group (ECOG) sta-tus.19 BCLC staging classification is based on these factors to stratify patients in 5 stages (0, A, B, C and D) and to allocate therapies according to the stage.4 According to European Association of Study of Liver recommenda-tions,18 TACE is first line-therapy in intermediate HCC BCLC stage B and sorafenib in advanced HCC BCLC stage C.
The rationale for TACE is intra-arterial infusion of a cytotoxic agent followed by embolization of the tumor-feeding blood vessels with an aim to achieve strong cy-totoxic and ischemic effect on the tumor cells.20 Hence it is the best palliative option for intermediate stage HCCs that are not amenable to curative resection or transplantation.9 Secondly it is also used as a bridge to liver transplantation to downstage or maintains lesions within the transplant criteria while waiting for a deceased donor liver.6 Thirdly, TACE has a potential therapeutic role as an adjuvant procedure for post operative tumor recurrence.7
In our cohort, we found that addition of sorafenib to TACE improved median survival from 9 months to 16 months in BCLC stage B group (p < 0.05). Similarly, combination of TACE and sorafenib improved median survival from 4 months to 9 months in BCLC stage C group (p < 0.0001). We also found that there was significant improvement in tumor morphology in the form of better partial response and reduction in incidence of progressive disease with combination of TACE and soraf-enib compared to sorafenib alone in BCLC stage C. There was modest improvement in tumor response with addition of sorafenib to TACE in BCLC stage B group as well but not statistically significant. This implies that loco-regional therapy like TACE is essential to enhance tumor response as it directly instills chemotherapeutic agent into the tumor cells followed by blockage of the feeding arterial vessel, which culminates in tumor hy-poxia. Sorafenib, as a multi-kinase inhibitor, delays the tumor progression in HCC patients by inhibiting tumor cell proliferation and neoangiogenesis.8 Local treatments including TACE, surgery, or radiofrequency ablation, can induce the overproduction of vascular endothelial growth factor, which may promote disease progression or metastasis.9 Therefore, sorafenib, as complementary treatment acting on vascular endothelial growth factor (VEGF), may enhance the treatment outcomes by reducing VEGF over expression, when administered sequentially after TACE.
Preliminary results of phase II START study21 in a subgroup analysis of Chinese patients had shown that combination of TACE with sorafenib to have a promising role in terms of efficacy and safety in BCLC stage B HCC. The study enrolled patients with unresectable HCC on TACE with sorafenib. The treatment protocol was use of lipi-odol and doxorubicin (30-60 mg) once in 6-8 weeks in combination with sorafenib 400 mg bid given daily. Soraf-enib was discontinued 4 days before and after TACE. Complete response rate was seen in 30% and a partial or stable disease rate in 60% with no major adverse effects. Results were similar to this in another subgroup analysis of the START trial among patients in Asia-Pacific re-gion.22 There was improved overall survival and time to progression with combination of TACE and sorafenib in a meta-analysis in 1254 patients with intermediate HCC.11 However, the recently published multi-centric STORM trial in 1602 patients showed that addition of sorafenib to resection or ablation in early HCC did not improve recurrence free survival.12 Hoffmann, et al.23 reported similar results in patients waiting for liver transplantation. In the recently published SPACE trial, use of sorafenib in combination with doxorubicin-eluting beads in TACE had no overall benefit in BCLC stage B patients.24
There are no studies that have used a combination of TACE with sorafenib in BCLC stage C group till date. In our series, while majority had either a partial response or progressive disease with very few having a stable disease, the survival benefit increased from 4 months to 9 months. None of the patients had any major adverse events. 80% patients had post TACE syndrome which improved with supportive measures. Hand foot syndrome was commonly reported with use of sorafenib which improved with dose reduction and local application of epsum salt.
The possible limitations of the study are limited number of patients, single center experience, effect of combination of TACE and sorafenib on tumor markers like AFP was not assessed. Hence further large prospective multicentric studies are needed to validate the use of combination of TACE and sorafenib especially in advanced hepatocellular carcinoma BCLC stage C patients.
ConclusionOur study has certainly shown that addition of soraf-enib to TACE in BCLC stage B patients and TACE to sor-afenib in BCLC stage C patients offered significant survival benefit and was associated with a significant improvement in tumor morphology especially in BCLC stage C patients with a good safety profile.
Abbreviations- •
BCLC: Barcelona Clinic Liver Cancer classification.
- •
HCC: hepatocellular carcinoma.
- •
MELD: Model for End-stage Liver Disease.
- •
mRECIST: Modified Response Evaluation Criteria In Solid Tumors.
- •
TACE: transarterial chemo-embolization.
We thank Mr Tom Michael, Jr Research Fellow, Mr Britzer Paul, Research officer and Dr. Sukanya Lakshmi, Duty medical officer for technical assistance.
Potential Conflicts of InterestNone.
FundingNone.
Author ContributionsJoy V, JV and VS made the study concept and design; acquisition of data done by Joy V, TD, MCU and MSR; analysis and interpretation done by JV and VS; drafting of manuscript done by Joy V and JV; critical revision of manuscript done for important intellectual content done by CKK and MR.