This study aims to investigate the antiviral effect of polyethylene glycol (PEG)-interferon α-2a and PEG-interferon α-2b treatment on hepatitis B e antigen (HBeAg)-positive chronic hepatitis B (CHB) at the 48th week of treatment and the 24th and 48th week after withdrawal, in order to provide guidance on the antiviral treatment of HBeAg-positive CHB patients.
Material and methodsAntiviral treatment was performed on 155 HBeAg-positive CHB patients. Among these patients, 66 patients received PEG-interferon α-2a treatment and 89 patients received PEG-interferon α-2b treatment; and these treatments were administered by subcutaneous injection, once per week, which lasted for 48 weeks. Other antiviral and hepatoprotective drugs were not used during the treatment.
ResultsAt the 48th week of treatment, ALT recovery rate, HBsAg seroconversion rate, HBeAg seroconversion rate and HBV DNA titers dropped below 200 IU/mL rate were 69.7%, 6.1%, 27.3% and 50.0%, respectively, in the PEG-interferon α-2a group; and were 70.8%, 6.7%, 33.7% and 62.9%, respectively, in the PEG-interferon α-2b group. At the 24th and 48th week of follow-up after withdrawal, HBsAg seroconversion rate in these two groups did not change; and HBeAg seroconversion rate further increased. Furthermore, HBV DNA revealed a low recurrence rate. The difference between these two groups was not significantly significant.
ConclusionsPEG-interferon α-2a and PEG-interferon α-2b are effective antiviral drugs for the treatment of HBeAg-positive CHB, which has a HBsAg seroconversion rate of more than 5%. Furthermore, this sustained response effect was maintained at the 24th and 48th week of follow-up after withdrawal.
CHB remains a global public health problem,1-3 and China currently has the largest number of individuals infected with hepatitis B virus (HBV) in the world. Clinical reports4 have revealed that CHB patients do not generally manifest any specific symptom. However, the incidence of liver cirrhosis and liver cancer remains high, and is considered a serious threat to the life and health of patients. At present, there is currently no existing drug that can thoroughly cure hepatitis B.5-7 As pointed out by the 2010 Guidelines for the Prevention and Treatment of CHB,8 if CHB patients in the active phase show indications, standard anti-HBV treatment should be performed. This treatment includes interfer-on and nucleoside drugs. Furthermore, based on the guidelines of the American Association for the Study of Liver Diseases (AASLD), PEG-interferon α is recommended as the first-choice drug when taking into account the long-term nature of antiviral therapy and the risk of drug-resistance in long-term treatment.9 In this study, we retrospectively investigated the antiviral effect of PEG-interferon α-2a and PEG-interferon α-2b for treating HBeAg-positive CHB, in order to provide guidance on antiviral treatment for HBeAg positive CHB patients.
Materials and MethodsStudy subjectsA total of 155 HBeAg-positive CHB patients in the outpatient or inpatient of our hospital from January 2011 to June 2012 were enrolled according to the following criteria:
- •
Antiviral treatment: Positive HBeAg patients, HBV DNA >20,000IU/mL; ALT Continuous Elevation >2 × ULN (more than 3 months) and <10 x ULN, serum total bilirubin < 2 x ULN.
- •
Patients who never used anti-HBV drugs.
- •
Age: 8-50 years old.
- •
Patients who are willing to use peg-IFN cc-2a or PEG-interferon cc-2b and signed informed content.
Meanwhile, the following conditions were excluded:
- •
Patients who have absolute or relative contraindications for interferon.
- •
Patients who suffered liver cirrhosis and other serious organ diseases.
- •
Patients who can not bear adverse reaction of interferon. Among these patients, 66 patients received PEG-interferon cc-2a treatment.
Among these 66 patients, 52 patients were male and 14 patients were female; and the age of these patients ranged between 17-48 years old, with an average age of 29.61 ± 7.91 years old. These patients were given a dose of 135 (Xg/ week. The remaining 89 patients received PEG-interferon cc-2b treatment. Among these patients, 69 patients were male and 20 patients were female; nd the age of these patients ranged between 9-43 years old, with an average age of 26.67 ± 7.06 years old. These patients were given a dose of 1-1.5 (Xg/kg/week. All treatment protocols were administered through subcutaneous injection, which lasted for 48 weeks. Other antiviral and hepatoprotective drugs were not used during the treatment. These selected patients met the interferon therapy indications written in the Guidelines for the Prevention and Treatment of CHB, and were diagnosed with HBeAg-positive CHB.
Detection indexDuring treatment, the following items were regularly monitored: blood routine, liver (“ALT recovery” means that ALT comes to the normal level, < 50U/L) and kidney function, myocardial enzyme spectrum, electrolyte, serum HBV markers, HBV DNA (“HBV DNA titers dropped below 200 IU/mL” means that HBV DNA comes to the negative, < 200 IU/mL), and thyroid function. Serum HBV markers in all patients were quantitatively determined by electrochemiluminescence using the Architect i2000 Immunoassay Analyzer (Abbott Laboratories, USA), HBsAg was tested with chemiluminescence. Serum DNA HBV quantitation was conducted using a PE5700 PCR amplification instrument (a result < 200 IU/mL was determined as negative).
Statistical methodsData was analyzed using statistical software SPSS 11.5. Measurement data were expressed as mean ± standard deviation (x ± SD). χ2 test was used to compare count data between these two groups, comparison of age, ALT and HBV-DNA by t test, and P < 0.05 was considered statistically significant.
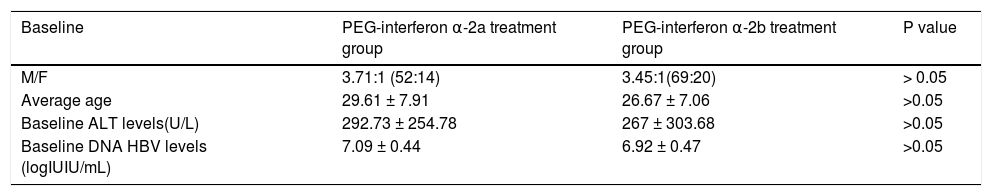
ResultsComparison of baseline situations between these two groups of patientsBaseline situations of patients in the PEG-interferon α-2a and PEG-interferon α-2b groups: Differences in male-to-female ratio, average age, baseline ALT levels and baseline DNA HBV levels between these two groups was not statistically significant (P > 0.05). These results are shown in table 1.
Comparison of baseline in two groups.
| Baseline | PEG-interferon α-2a treatment group | PEG-interferon α-2b treatment group | P value |
|---|---|---|---|
| M/F | 3.71:1 (52:14) | 3.45:1(69:20) | > 0.05 |
| Average age | 29.61 ± 7.91 | 26.67 ± 7.06 | >0.05 |
| Baseline ALT levels(U/L) | 292.73 ± 254.78 | 267 ± 303.68 | >0.05 |
| Baseline DNA HBV levels (logIUIU/mL) | 7.09 ± 0.44 | 6.92 ± 0.47 | >0.05 |
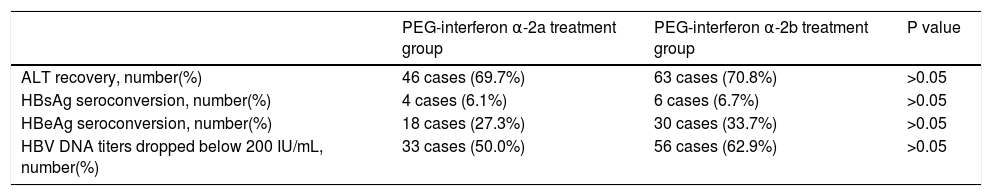
At the 48th week of treatment, ALT recovery rate, HB-sAg seroconversion rate, HBeAg seroconversion rate and HBV DNA titers dropped below 200 IU/mL rate were 69.7%, 6.1%, 27.3% and 50.0%, respectively, in the PEG-in-terferon α-2a group; and were 70.8%, 6.7%, 33.7% and 62.9%, respectively, in the PEG-interferon α-2b group. Overall response rate was higher in the PEG-interferon α-2b group than in the PEG-interferon α-2a group. However, the difference between these two groups was not significantly significant (P > 0.05). These results are shown in table 2.
Response in patients in these two groups at the 48th week of treatment.
| PEG-interferon α-2a treatment group | PEG-interferon α-2b treatment group | P value | |
|---|---|---|---|
| ALT recovery, number(%) | 46 cases (69.7%) | 63 cases (70.8%) | >0.05 |
| HBsAg seroconversion, number(%) | 4 cases (6.1%) | 6 cases (6.7%) | >0.05 |
| HBeAg seroconversion, number(%) | 18 cases (27.3%) | 30 cases (33.7%) | >0.05 |
| HBV DNA titers dropped below 200 IU/mL, number(%) | 33 cases (50.0%) | 56 cases (62.9%) | >0.05 |
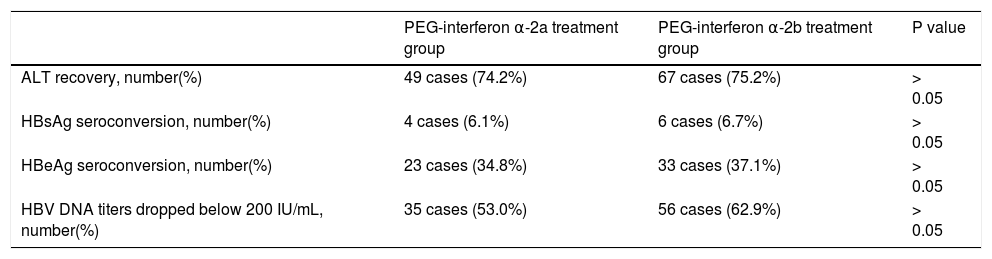
At the 48th week of treatment, ALT recovery rate, HBsAg seroconversion rate, HBeAg seroconversion rate and HBV DNA titers dropped below 200 IU/mL rate were 74.2%, 6.1%, 34.8% and 53.0%, respectively, in the PEG-interferonα-2agroup;andwere75.2%,6.7%,37.1% and62.9%,respectively,inthePEG-interferonα-2b group. This response effect was maintained in these two groups of patients at the 48th week after withdrawal. These results are shown in table 3.
Response situations in patients in these two groups at the 48th week after drug withdrawal.
| PEG-interferon α-2a treatment group | PEG-interferon α-2b treatment group | P value | |
|---|---|---|---|
| ALT recovery, number(%) | 49 cases (74.2%) | 67 cases (75.2%) | > 0.05 |
| HBsAg seroconversion, number(%) | 4 cases (6.1%) | 6 cases (6.7%) | > 0.05 |
| HBeAg seroconversion, number(%) | 23 cases (34.8%) | 33 cases (37.1%) | > 0.05 |
| HBV DNA titers dropped below 200 IU/mL, number(%) | 35 cases (53.0%) | 56 cases (62.9%) | > 0.05 |
HBV infection-related liver failure, cirrhosis and primary liver cancer are a serious threat to the life of patients. Theanti-HBVmechanismofinterferon-α includesdirect antiviral effect and immunoregulation.10-12 Interferon-a can inhibit viral DNA replication, degrade viral RNA, inhibit the synthesis and transport of viral protein, and block the secretion of mature virus. In addition, its immunolog-ical mechanism is more complicated. PEG-interferon a derivesfrominterferon-α throughtheadditionofalarge volume of branched PEG molecules, which increases the molecular weight of interferon and reduces drug excretion, shields the antigenic determinant on the surface of interferon molecules, and reduces immunogenicity and the clearance rate of interferon in the body. Furthermore, it slows down the hydrolysis of protease, in which its half-life can be prolonged up to 40 h. This enables interferon to continuously work in the body, and effectively improves the antiviral effect.13 In addition, PEG-interferon is mainly metabolized in the liver. Hence, it can selectively exert on the target organs of hepatitis, playing the dual role of immunoregulation and antiviral action. It was reported by Zhe Li14 that in PEG-interferon treatment for hepatitis B, HBeAg seroconversion rate could reach 48%, HBV DNA titers dropped below 200 IU/mL rate was more than 50%, ALT and AST recovery rate was up to 35%, histologi-cal improvement rate was 67%, and 9% of the patients underwent HBsAg-negative conversion. Through studies, some Chinese scholars considered that the clinical effect of long-term interferon was better than that of conventional interferon.15-17 Phase II clinical trials have revealed thatPEG-interferonα-2aissuperiortoconventionalin-terferon in the treatment of HBeAg-positive CHB.18 The study of Chan, et al. demonstrated19 that HBsAg in 5% (2/ 40) of CHB patients became negative after PEG-interfer-onα-2atreatment.Pisit,et al.20reportedthat48weeksafter PEG-interferonα-2btreatment,33.3%HBeAg-positive CHB patients underwent HBeAg seroconversion and HBV DNA negative conversion. However, there is currently no large-sample controlled study in China. In this study,66patientsreceivedPEG-interferonα-2atreatment and89patientsreceivedPEG-interferonα-2btreatment. Differences in male-to-female ratio, average age, baseline ALT levels and baseline DNA HBV levels between these two groups were not statistically significant (P > 0.05). At the 48th week of treatment, ALT recovery rate, HBsAg se-roconversion rate, HBeAg seroconversion rate and HBV DNA titers dropped below 200 IU/mL rate were 69.7%, 6.1%, 27.3% and 50.0%, respectively, in PEG-interferon α-2agroup;andwere70.8%,6.7%,33.7%and62.9%, respectively,inthePEG-interferonα-2bgroup.Theseresults demonstrated that at the 48th week of treatment for HBeAg-positiveCHB,bothPEG-interferonα-2aand PEG-interferonα-2brevealedahigherantiviralresponse. HBsAg seroconversion rates were all over 5%. Overall responseratewashigherinthePEG-interferonα-2bgroup thaninthePEG-interferonα-2agroup,butthedifference between these two groups was not significantly significant (P > 0.05). An international multicenter randomized controlled clinical trial has revealed that in HBeAg-positive CHB patients who received 48 weeks of PEG-interferon treatment, HBeAg serum conversion rate was 43% at the 48th week follow-up after withdrawal. Results of this study revealed that in these two groups of patients, at the 48th week follow-up, HBsAg seroconversion rates did not change, HBeAg seroconversion rate further increased, and HBV DNA exhibited a low recurrence rate; suggesting thattheresponseeffectofPEG-interferonα wassustained after withdrawal, and difference between the two groups was not significantly significant.
Throughthisstudy,PEG-interferonα-2aandPEG-in-terferonα-2bwerefoundtobeeffectivefortreating HBeAg-positive CHB, with a HBsAg seroconversion rate of more than 5%, and a sustained response effect at the 48th week follow-ups after withdrawal. HBeAg positive CHB patients, who have conditions, should give priority to PEG-interferon a treatment.
Conflict of InterestNone.