We report a case of new interferon-associated ocular complication during treatment with combination of pegylated interferon plus ribavirin for chronic hepatitis C infection. Our patient developed choroidal neovascularization in addition to the classic interferon associated retinopathy. Choroidal neovascularization has not been reported before in association with interferon induced retinopathy. We describe our management to control the ocular symptoms and the retinal lesions with one year follow up. We also provide literature report on the natural history, the pathophysiology and the variable characteristics of interferon associated retinopathy versus hepatitis C related ophthalmopathy.
Abbrivations:
HCV hepatitis C virus
VAL visual acuity of left eye
VAR visual acuity of right eye
IntroductionSince interferon-α became available for the treatment of hepatitis C infection, constitutional symptoms as well as complications involving almost every single system have become recognized side effects of this therapy.1 Ocular complications of variable pathology and severity have been reported in association with interferon therapy.2
We report here a case with a new interferon-associated ocular complication in one of our patients who had been receiving combination therapy with pegylated interferon plus ribavirin for chronic hepatitis C. Our patient developed choroidal neovascularization on a background of the classic interferon-associated retinopathy. Choroidal neovascularization has not been reported before in association with interferon-associated retinopathy. We summarize the presenting symptoms and management of the patient’s retinopathy with over one year follow up. We also review the literature on the natural history, pathophysiology, and characteristics of both ocular manifestations of hepatitis C viral infection and interferon-associated retinopathy.
Case reportOur patient was 42-year old Caucasian female chronically infected with hepatitis C genotype 3a virus. She had acquired hepatitis C through remote injection drug misuse. Past medical history, and physical examination were unremarkable. Specifically, there were no visual disturbances, no history of diabetes mellitus or hypertension, and her CBC, platelet count, INR, PTT, lipid profile, and cryoglobulin levels were all within normal range. In the absence of contra-indications, she was started on 6-month combination therapy of pegylated interferon-α and ribavirin according to the Canadian guidelines.3
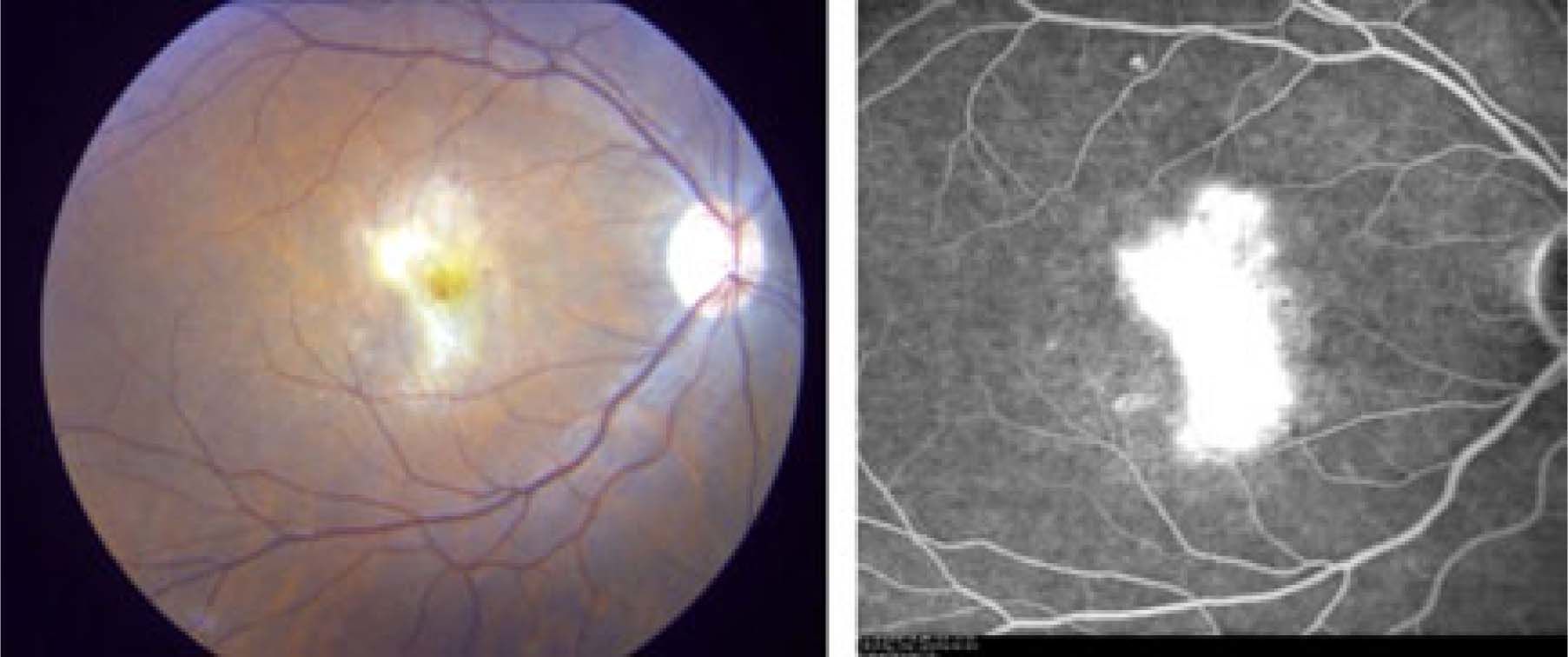
Seventeen weeks post initiation of therapy she developed bilateral visual distortion with reduced visual acuity in the right eye. Urgent consultation with ophthalmology revealed central visual distortion. Her VAR was 6/7.5 and VAL was 6/6, both pupils were normal. Retinal examination revealed retinopathy with cotton wool spots, hemorrhages and multi-focal choroidal neovascularization (confirmed by intravenous fluorescein angiography) both superior and inferior to the right fovea (Figure 1)
Interferon-associated retinopathy was suspected. Pegy-lated interferon and ribavirin were discontinued immediately. Investigations for other causes of retinopathy were negative including: fasting blood glucose 5.1 mmol/L, HgbA1c 5.0%, negative Tuberculin PPD skin test, negative serology for Herpes and Human Immunodeficiency viruses, Toxocara, and Toxoplasmosis. Cytomegalovirus serology was positive for IgG and negative for IgM indicating remote exposure to this virus but no active infection.
The patient was commenced on prednisone 60 mg daily with a rapid tapering of the dose at 5 mg every 3 days. At follow up three weeks later, the patient was taking prednisone 30 mg daily. She reported a dramatic improvement in her visual symptoms; her VAR was 6/6 and VAL was 6/6. Examination of her retina demonstrated impressive early involution of the lesions seen on presentation (Figure 2)
Eight weeks after start of prednisone, the patient reported recurrent awareness of visual distortion. She was taking prednisone 5 mg daily; on examination at this time, her VAR was 6/7.5 and VAL was 6/6, but there were slight worsening of the retinal lesions (Figure 3) The dose of prednisone was increased to 50 mg daily for one week and visudyne photodynamic therapy was carried out to the area of macular retina affected by choroidal neovascularization.
Subsequent follow up revealed fluctuations with intermittent recurrence of symptoms, complete resolution of retinopathy lesions, but incomplete resolution of choroidal neovascularization with intermittent flare up of these lesions (Figures 4-5).
During the time when the patient was on prednisone, we did not see any clinical or biochemical evidence of hepatitis flare-up. Although her hepatitis C viral RNA was non-detectable at 17-weeks, she was again viremic at 24-weeks. We decided not to re-treat her with interferon-α and ribavirin but wait. She has ongoing viremia with hepatitis C genotype 3a. A liver biopsy one year after the cessation of interferon-α and ribavirin revealed minimal activity with peri-portal fibrosis (METAVIR score: grade-1; stage-2).
DiscussionOur patient clearly had interferon-associated retinopathy which remitted upon cessation of pegylated interferon and ribavirin as evidenced by clinical improvement and ophthalmic examination. This is in agreement with published reports4,5 that retinopathy is a recognized complication of interferon therapy that is occurring at a frequency slightly higher than it was thought to be. In addition, our patient also developed progressive choroidal neovascularization necessitating early intervention with systemic steroids and photodynamic therapy. Choroidal neovascularization has not been reported as part of interferon-associated retinopathy.
Whether systemic steroids are needed to induce or maintain remission of interferon-associated retinopathy is poorly documented, and remains controversial in view of its possible down side for flaring up viral hepatitis. The rapid progression of her visual distortion and decreased acuity as well as the retinal findings of choroidal neovascularization with hemorrhage in the right fovea were behind our decision to treat with steroids rather than wait for spontaneous resolution.
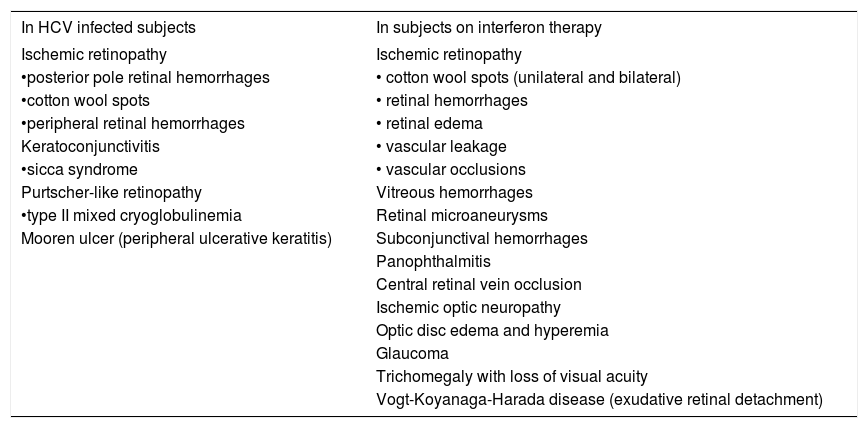
Immune system dysfunction, deposition of immune complexes in the retinal vessels and increased adhesions of activated leukocytes to vascular walls have all been postulated as possible mechanisms for interferon-associated retinopathy.6-8 The incidence of retinopathy has been reported to be dose dependent with no difference in the incidence of retinopathy observed between interferon-α and interferon-β, nor between hepatitis B and C when treated with interferons, including pegylated interferon-α with or without ribavirin. Diabetes mellitus is recognized as a strong risk factor for the development and progression of interferon-associated retinopathy.6,9 Other risk factors include: older age, hypertension, decreased platelet count and increased triglyceride levels.10 Hypoalbuminemia was implicated for cystoid macular edema reported in some cases.11 Whether hepatitis C virus itself predisposes or causes retinopathy remains unclear although it has been reported by some authors.12Table I summarizes the ocular manifestations of hepatitis C virus and the visual side effects of interferon during therapy for chronic hepatitis C infection.
Reported ocular complications potentially associated with hepatitis C viral infection or with interferon therapy.
| In HCV infected subjects | In subjects on interferon therapy |
|---|---|
| Ischemic retinopathy | Ischemic retinopathy |
| •posterior pole retinal hemorrhages | • cotton wool spots (unilateral and bilateral) |
| •cotton wool spots | • retinal hemorrhages |
| •peripheral retinal hemorrhages | • retinal edema |
| Keratoconjunctivitis | • vascular leakage |
| •sicca syndrome | • vascular occlusions |
| Purtscher-like retinopathy | Vitreous hemorrhages |
| •type II mixed cryoglobulinemia | Retinal microaneurysms |
| Mooren ulcer (peripheral ulcerative keratitis) | Subconjunctival hemorrhages |
| Panophthalmitis | |
| Central retinal vein occlusion | |
| Ischemic optic neuropathy | |
| Optic disc edema and hyperemia | |
| Glaucoma | |
| Trichomegaly with loss of visual acuity | |
| Vogt-Koyanaga-Harada disease (exudative retinal detachment) |
Our patient was treated initially with prednisone 60 mg daily for a week then tapered by 5 mg every third day. Certainly, we observed a definite relapse in the clinical symptoms, visual acuity and retinopathy lesions (Figure 3) in our patient when the steroid dose was tapered rapidly after an initial impressive improvement when they were first started. This relapse was brought under control by raising the steroid dose, and tapering it down slowly. Prednisone was then increased to 50 mg daily for a week and tapered by 5 mg every week. Complete remission of retinopathy was achieved and sustained. However, this was not the case with the choroidal neovascularization.
Our patient had choroidal membrane neovascular complex in both the superior and inferior aspects of her right fovea. This initially resolved on treatment with systemic steroids and photodynamic therapy (Figure 2) Follow up revealed fluctuations in the neovascular choroidal lesions despite slow tapering of prednisone dose; this was also associated with deterioration of the visual acuity in the affected right eye. Whether longer term steroids would have made any difference is unknown. Our patient had none of the risk factors or identifiable etiologies for choroidal neovascularization; pegylated interferon-á remains a possible causative factor.
Literature reviewInterferon has multiple clinical usage including as an antiviral therapy,13 and tumor suppressor activity in human neoplasm.14 It has also been used to treat a variety of ocular disorders including dendritic keratosis,15 subretinal neovascularization in age related macular degeneration,16 ocular cicatrical pemphigoid,17 and for glaucoma filtering surgery.18
With increased clinical use of interferon in treating a variety of conditions including hepatitis C infection, a wide spectrum of side effects have become increasingly recognized, ranging from constitutional symptoms with flu-like syndrome manifested as fever, myalgia, arthralgia, and headache being the most common acute side effect to chronic side effects such as fatigue, as well as variable degrees of organ specific toxicities involving almost any organ including gastrointestinal, hematopoietic, renal, skin, musculoskeletal, cardiovascular, endocrine and central nervous system.
Ocular complications of interferon have only been recognized increasingly in recent years since the first report was in 1990 where a Japanese young woman developed retinopathy following administration of intravenous interferon.19
Interferon-associated retinopathy is typically characterized by retinal hemorrhages and cotton wool spots occurring in combination or alone.5,6,19 As summarized in Table I several other less common ocular side effects have also been described including oculomotor nerve paralysis, optic disc edema, and retinal vein occlusion, in addition to subconjunctival, preretinal or vitreous hemorrhage.5 Retinal microaneurysms have also been reported.5 Our patient had choroidal neovascularization of the choroidal membrane of the right macula which has not been reported in association with regular dose interferon therapy, or with the typical interferon-associated retinopathy.
Retinopathy usually develops between 3 weeks to four months from starting interferon therapy. It may disappear spontaneously during therapy or rapidly after stopping therapy.5,17,19 Slow progression of the retinopathy after discontinuing interferon, but with eventual complete resolution has also been reported.19
ConclusionRetinopathy is a recognized complication of interferon therapy but not reported with ribavirin. This case is unusual since choroidal neovascularization occurred during interferon therapy on a background of the more typical interferon-associated retinopathy. Although spontaneous resolution may occur (with or without interferon discontinuation),20 we recommend holding the interferon therapy once retinopathy is symptomatic and progressive with hemorrhage. The use of high dose steroids with slow tapering should be based on the type and severity of retinal lesions as well as progression of clinical symptoms. Certainly in case of neovascularization as with our patient, steroids appeared to be effective and beneficial without flare-up of hepatitis C viral infection.
We do not think that screening all patients for retinopathy prior to starting interferon is justifiable.21 Yet, careful inquiry about any visual disturbances or risk factors together with documentation of patient’s baseline visual acuity is necessary prior to starting interferon therapy. Enquiry about visual symptoms during follow up visits should also beencouraged and their presence should lead to ophthalmological assessment with a focus on the retina. Patients who have visual problems prior to therapy or risk factors such as diabetes or hypertension as well as congenital or family history of significant visual disorder should be referred to have a complete baseline ophthalmological assessment followed by regular monitoring.