We read with interest the article by Mendizabal et al. [1], which mainly evaluated the association of abnormal liver tests at admission with clinical outcomes in coronavirus disease 2019 (COVID-19) patients. COVID-19 mainly leads to acute respiratory symptoms, including fever, dry cough, and dyspnea. Besides, 16–76% of COVID-19 patients manifest as varying degrees of abnormal liver biochemistry tests [2–4], which are secondary to viral infection of liver cells, liver hypoxia, drug-induced liver injury, and immune-inflammatory reaction [5,6]. Notably, a minority of them may progress into liver failure [7]. Some studies suggested that abnormal liver biochemical tests, especially elevated aspartate aminotransferase (AST), be closely associated with severe outcome [2,8] and death [9,10] in COVID-19 patients. Contrarily, others indicated no significant correlation of abnormal liver biochemical tests with mortality [11] or disease severity [12]. The current study aimed to explore the incidence and outcomes of abnormal liver biochemical tests in a large cohort of COVID-19 patients.
All study investigators are from the General Hospital of Northern Theater Command in Shenyang, and some of them had voluntarily joined in the clinical management of COVID-19 patients at the Huoshenshan Hospital in Wuhan from February 2020 to April 2020. The current study retrospectively reviewed the medical records of 3041 patients with COVID-19 consecutively admitted to the Huoshenshan hospital during this period. Exclusion criteria were as follows: (1) patients were lacking of liver function tests at admission and during hospitalization; and (2) patients were previously diagnosed with hepatobiliary diseases, including chronic liver disease, cirrhosis, liver cancer, and bile duct obstruction. This study protocol was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command (Ethical Approval No. Y [2020] 062) and performed according to the Declaration of Helsinki.
Diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program published by the National Health Commission of China. Severity of COVID-19 patients was classified into mild, moderate, severe or critical [13].
Liver biochemical abnormality was defined as at least one of liver-related biochemical tests exceeding upper limit of normal of reference range at Huoshenshan hospital (i.e., males: AST > 60 U/L, alanine aminotransferase [ALT] > 50 U/L, total bilirubin [TBIL] > 26 μmol/L, alkaline phosphatase [ALP] > 125 U/L, gamma-glutamyl transpeptidase [GGT] > 60 U/L, albumin [ALB] < 40 g/L; females: AST > 45 U/L, ALT > 40 U/L, TBIL > 21 μmol/L, ALP > 135 U/L, GGT > 45 U/L, ALB < 40 g/L). Liver biochemical indicators at admission and during hospitalization referred to laboratory values obtained within and after the first 24 h during hospitalization, respectively.
The composite end point was considered as requirement of ICU management or mechanical ventilation or death.
After excluding 244 COVID-19 patients with hepatobiliary diseases or those who were lacking of liver function tests, 2797 patients were finally included. The mean age was 60 years (range 11–100), and 50.1% were male. Hypertension (32%) and diabetes (14.7%) are common comorbidities. At admission, 730 (26.1%) and 42 (1.5%) patients were severe and critical, respectively. During hospitalizations, 2.4% (65/2749) patients progressed from mild/moderate to severe/critical cases, 3.1% (87/2797) were admitted to the ICU, 2.9% (82/2797) underwent mechanical ventilation, and 2.3% (63/2797) died, and 4.5% (125/2797) met the composite endpoint.
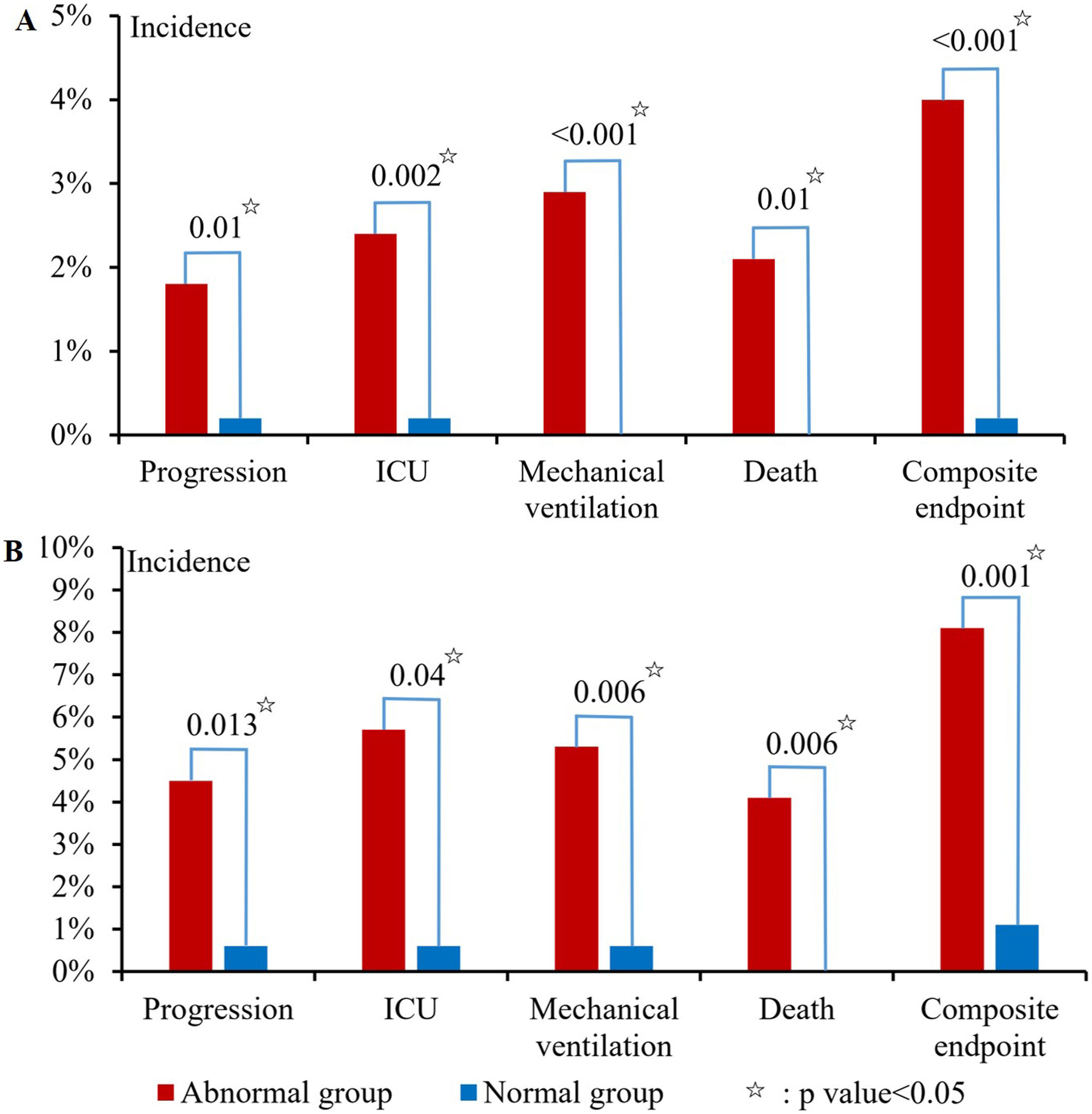
At admission, 76.5% (1594/2083) of patients had abnormal liver biochemical tests, of which abnormal ALB was the most common, followed by GGT, ALT, AST, ALP, and TBIL (65.1%, 20.4%, 14.8%, 4.4%, 3.5%, and 2.5%). Abnormal liver biochemical tests at admission were significantly associated with disease progression (p = 0.01), ICU (p = 0.002), mechanical ventilation (p < 0.001), death (p = 0.01), and composite endpoint (p < 0.001) (Fig. 1A).
During hospitalization, 89.4% (1463/1637) of patients had abnormal liver biochemical tests, of which abnormal ALB was the most common, followed by GGT, ALT, AST, ALP, and TBIL (83.9%, 30.5%, 29.6%, 10%, 7.9%, and 3.5%). Abnormal liver biochemical tests during hospitalization were significantly associated with disease progression (p = 0.013), ICU (p = 0.04), mechanical ventilation (p = 0.006), death (p = 0.006), and composite endpoint (p = 0.001) (Fig. 1B).
The present study showed that liver biochemical abnormality was common in COVID-19 patients, especially during hospitalization. Except for the infection of this virus itself and disease progression, it may be partly due to the use of multiple drugs, such as empirical antibiotics or antiviral therapy, which are potentially hepatotoxic. The present study also indicated that patients with liver biochemical abnormality were at an increased risk of poor prognosis. Therefore, liver biochemistry in COVID-19 patients should be screened at admission and intensely monitored and managed during hospitalization. A limitation should be acknowledged that severe or critical COVID-19 patients are more likely to have repeated laboratory tests and diagnose with liver injury. In future, prospective studies are needed to explore whether the management of abnormal liver biochemical indicators will influence the patients' outcomes.
Financial supportNone.
Conflict of interestThe authors declare that there is no conflict of interest in this study.
Availability of data and materialThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AcknowledgmentsWe are indebted to all of the medical staffs who volunteered to participate in the treatment of COVID-19 patients at the Wuhan Huoshenshan Hospital. We would like to appreciate our study team for collecting the data of COVID-19 patients, including Yang An, Li Luo, Yiyan Zhang, Haijuan, Yao, Fangfang Yi, and Hongxin Chen.