Bacterial infections are associated with a dismal prognosis in patients with liver cirrhosis. Data on their prevalence and the associated pathogen spectra in Germany are scarce. This study aimed to evaluate the impact of bacterial infections on mortality in hospitalized patients with liver cirrhosis and to analyze the prevalence of multidrug-resistant (MDR) bacteria in a German tertiary care center.
Patients and MethodsConsecutive, non-electively hospitalized patients with liver cirrhosis were enrolled in this study between 03/2019-06/2021. All patients underwent clinical, laboratory and microbiological testing to detect potential bacterial infections. Patients were followed for 30 days regarding the composite endpoint of death or liver transplantation (mortality).
ResultsIn total, 239 patients were recruited (median MELD 18). Bacterial infection was detected in 81 patients (33.9%) at study inclusion. A total of 70 patients (29.3%) developed a hospital-acquired infection. When comparing community-acquired and hospital-acquired infections, the pathogen pattern shifted from a gram-negative to a more gram-positive spectrum and showed an increase of Staphylococcus spp.. MDR bacteria were detected in seven infected patients (5.8%). 34 patients reached the composite endpoint during 30-days follow-up. In multivariable logistic regression analysis, the presence of infection during hospitalization remained independently associated with higher mortality (OR 2.522, 95% CI 1.044 - 6.091, p = 0.040).
ConclusionsThis study demonstrates that bacterial infections are common in hospitalized patients with liver cirrhosis in Germany and are a major determinant of short-term mortality. Our data highlight the importance of regional differences in MDR bacteria and may guide physicians' decision-making regarding calculated antibiotic treatment.
Liver cirrhosis marks the common endpoint of almost all chronic liver diseases and accounts for more than one million deaths worldwide per year [1]. More than 25% of all hospitalized patients with decompensated liver cirrhosis suffer from bacterial infections during their hospital stay [2]. Infections themselves, in turn, are well-known triggers of cirrhosis-related complications and acute-on-chronic liver failure (ACLF) [3]. The most frequent infections are spontaneous bacterial peritonitis (SBP), urinary tract infections (UTI), pneumonia, soft tissue infections, and spontaneous bacteremia. [4] Bacterial infections are associated with a poor short- and long-term prognosis in these patients, thus, early diagnosis is essential to improve their outcomes [4,5]. However, signs of infection may be subtle and not very specific in patients with liver cirrhosis, often preventing a fast diagnosis [6].
Several factors contribute to the susceptibility to bacterial infections in patients with liver cirrhosis: Alterations and excessive growth of gut microbiome combined with an impaired intestinal barrier function increase the likelihood of bacterial translocation [7]. In addition, liver cirrhosis leads to a progressive immune dysfunction (cirrhosis-associated immune dysfunction (CAID) [5], including both systemic inflammation and immunodeficiency [5]. In case of an additional bacterial infection, the cardiovascular response is inadequate and culminates in a rapid hemodynamic collapse [5]. Therefore, infections are often more severe in patients with liver cirrhosis [7–10].
To improve the prognosis of patients with liver cirrhosis, it is of utmost importance to identify potential risk factors for infections and to initiate adequate antibiotic treatment in patients with any signs of infections [11]. Here, the choice of antibiotics has to be guided by the local epidemiology of multidrug-resistant (MDR) bacteria. Recent studies indicated an increase of MDR bacteria from < 10% in 1998 - 2000 up to 23% in 2010 - 2011 [12]. In this context, a recently published global study estimated the rate of MDR bacteria to be as high as 36% globally, below 20% in the USA, and more than 70% in India [13]. However, studies investigating the prevalence of MDR bacteria in patients with liver cirrhosis in Germany are scarce. Therefore, this study aimed to evaluate the impact of bacterial infections on mortality in hospitalized patients with liver cirrhosis and to analyze the prevalence of MDR bacteria in a German tertiary care center.
2Material and methods2.1Study populationThis study was a retrospective analysis of a prospectively collected database. A total of 239 consecutive inpatients with an established diagnosis of liver cirrhosis were enrolled between March 2019 and June 2021 at the Cirrhosis Center Mainz (CCM) of the University Medical Center Mainz, Germany. All patients were hospitalized non-electively, most often due to complications of liver cirrhosis, or infections. All patients were included in the study at initial presentation only. Repeated presentations were not included. All data were recorded within the first 48 hours of hospitalization. The leading etiology of the underlying liver disease was determined according to clinical, serological and histological findings. Diagnosis of liver cirrhosis was established by histology, conclusive appearance in ultrasound or radiological imaging, endoscopic features of portal hypertension, and medical history. Blood biochemistry (bilirubin, albumin, international normalized ratio (INR), sodium, potassium, creatinine, c-reactive protein (CRP), white blood cell count (WBC), hemoglobin, and thrombocytes were assessed in all patients. Model for end-stage liver disease (MELD) and Child-Pugh (CP) scores were calculated to determine the severity of the underlying liver disease. Each patient was monitored during clinical routine for the development of infections during the hospital stay. ACLF was defined according to the EASL-CLIF recommendations [14,15]. Patients with known malignancies (including hepatocellular carcinoma (HCC)) were not approached for this study.
2.2Assessment of bacterial infectionsInfections that were detected at hospitalization (study inclusion) or infections that developed during hospitalization were recorded. All infections were classified based on the time point of their occurrence in community-acquired (< 48 h after admission) or nosocomial (> 48 h after admission). Microbiological isolates (blood cultures, urine cultures, cultures from punctures, stool samples), the number of infections caused by MDR bacteria as well as the location of colonization, and the respective antibiotic treatment regimen were recorded using medical charts review.
Criteria for defining infectious complications were based on current guidelines of the Association of the Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V.) and the European Association for the Study of Liver Diseases (EASL, Table 1) [16–22]. According to the local standard of care and on the discretion of the treating physician, all patients with cirrhosis and suspected infections should have at least a urine analysis, a chest X-ray, skin inspection, and, if possible, a diagnostic paracentesis. However, it has to be mentioned that this study reflects a real-life scenario and the aforementioned standard of care was not followed in all patients. Blood cultures were taken peripherally in two sets of aerobic/anaerobic bottles at different sites. If line infections were highly suspected, additional blood cultures were taken from central lines. Urine samples were collected from patients via clean catch, otherwise via urinary catheter if present. Diagnosis of other infections were made based on conventional criteria. The occurrence of first signs of infection were precisely documented in the context of hospital-acquired infections. Microbiological diagnostics, in particular bacterial cultures from blood, urine, drains or stool, were considered positive if the pathogen detection provided a conclusive picture with laboratory and clinical infection constellations. For example, positive blood cultures with the detection of classical skin commensals such as coagulase-negative staphylococci were evaluated as negative (contamination) if their cultivation took more than 12 hours and was only possible once.
Definitions of the infection sites.
The Antimicrobial Susceptibility Testing was evaluated according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [23,24]. Each susceptibility category (S: susceptible, standard dosing regimen; I: susceptible, increased exposure; R: resistant) is defined by breakpoints that are specific for each species and agent. Resistant results include intrinsic resistances as well as acquired resistances. Resistance classification is carried out almost entirely by phenotypic criteria and not only according to resistance mechanisms. Gram-negative bacteria with extended intrinsic resistance e.g. Stenotrophomonas maltophilia were also included. The isolated bacteria considered to be multidrug-resistant were: Methicillin-/Oxacillin-resistant Staphylococcus aureus (MRSA), Vancomycin- or Linezolid-resistant Enterococcus (VRE) and Gram-negative bacteria including those producing extended spectrum beta-lactamases (ESBLs) and others that are resistant to multiple classes of antimicrobial agents [25–28]. The group of multidrug-resistant Gram-negative bacteria included Enterobacteriaceae as well as non-fermenters such as Pseudomonas aeruginosa and Actinetobacter baumanii complex. The classification of multidrug-resistant Gram-negative bacteria was based on the "New classification for multidrug-resistant Gram-negative bacteria" of the Commission for Hospital Hygiene and Infection Prevention (KRINKO) at the Robert Koch Institute, Germany. Not all antibiotics are weighted equally in this classification. Only the clinically most important ones such as acyl ureidopenicillins, extended-spectrum cephalosporins, carbapenems, and fluoroquinolones are taken into account. For a resistance to them has therapeutic consequences [28]. Gram-negative bacteria were classified as MDR if they were resistant to at least three of the four classes of antibiotics mentioned above.
2.4Assessment of colonization of MDR bacteriaSwabs for multidrug-resistant germs were taken from patients who were either admitted to a sensitive area at the University Medical Centre (organ transplant wards or intensive care units) or who met the local requirements for smear testing. The latter corresponded to patients who were transferred from other medical facilities such as hospitals or nursing homes. Swabs were taken intranasal, pharyngeal, and rectal according to a standardized protocol and tested for the above mentioned pathogens (MRSA, VRE, and MDR Gram-negative bacteria).
2.5Follow-up evaluationAll study participants were followed-up by medical charts review during their hospital stay and for the following 30 days after study inclusion. The composite endpoint of death or liver transplantation was evaluated as the primary endpoint during follow-up. As patients with HCC were excluded, all patients who had received a liver transplantation during follow-up were transplanted due to final liver failure and were consequently treated as complete cases (= death). Thus, the combined endpoint of death or liver transplantation is referred to as mortality in the following sections of the manuscript.
2.6Ethical statementThe study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all study participants and the study was approved by the ethics committee of the Landesärztekammer Rheinland-Pfalz (837.052.12 [8153]).
2.7Statistical analysesAll analyses were performed using SPSS (IBM SPSS Statistics 27, Version 27.0.0.0) and Microsoft Excel. Quantitative data are expressed as medians with interquartile ranges (IQR). Pairwise comparisons for quantitative variables were performed with an unpaired t-test or with the Mann-Whitney U-test. Categorical variables are given as frequencies and percentages, respectively. For the comparison of two or more patient groups a chi-square test was applied. To assess predictors for the occurrence of the composite endpoint of death or liver transplantation (mortality), differences between patients who deceased within 30 days or who survived were analyzed by univariable analyses. Variables with p < 0.05 in the univariable analysis were subsequently considered in a multivariable logistic regression model. This model was built based on a stepwise variable selection procedure. Our data analysis is exploratory and thus no adjustments for multiple testing were performed. P-values < 0.05 were considered statistically significant.
3Results3.1Study cohortA total of 239 consecutive, non-electively hospitalized patients with liver cirrhosis were included in this study. The reasons for hospital admission were either an acute decompensation event (hydropic decompensation, hepatic encephalopathy, bleeding events such as variceal bleeding, acute kidney injury / hepatorenal syndrome), infections or a combination of these. Most patients were male (64.0%) and the most common underlying etiology of liver cirrhosis was alcohol-induced liver disease (62.3%). The median MELD score was 18 (IQR 12; 24) and 93 patients (39.3%) suffered from ACLF (grade 1) at study inclusion. In total, 121 patients had at least one infection during hospital stay. Of those, 81 (33.9%) had a community-acquired infection and 70 (29.3%) a hospital-acquired infection. No patient suffered from a viral infection. Additional baseline characteristics of the cohort are displayed in Table 2.
Demographics and clinical characteristics of the entire cohort and stratified by the presence of infections at the time of study inclusion.
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
ACLF: acute-on-chronic liver failure; CP: child pugh; CRP: c-reactive protein; HE: hepatic encephalopathy; HRS: hepatorenal syndrome, ICU: intensive care unit; IQR: interquartile range; INR: international normalized ratio; LTX: liver transplantation n: number; MELD: model for end-stage liver disease; NAFLD: non-alcoholic fatty liver disease; SBP: spontaneous bacterial peritonitis; wbc: white blood cell; Yr: years
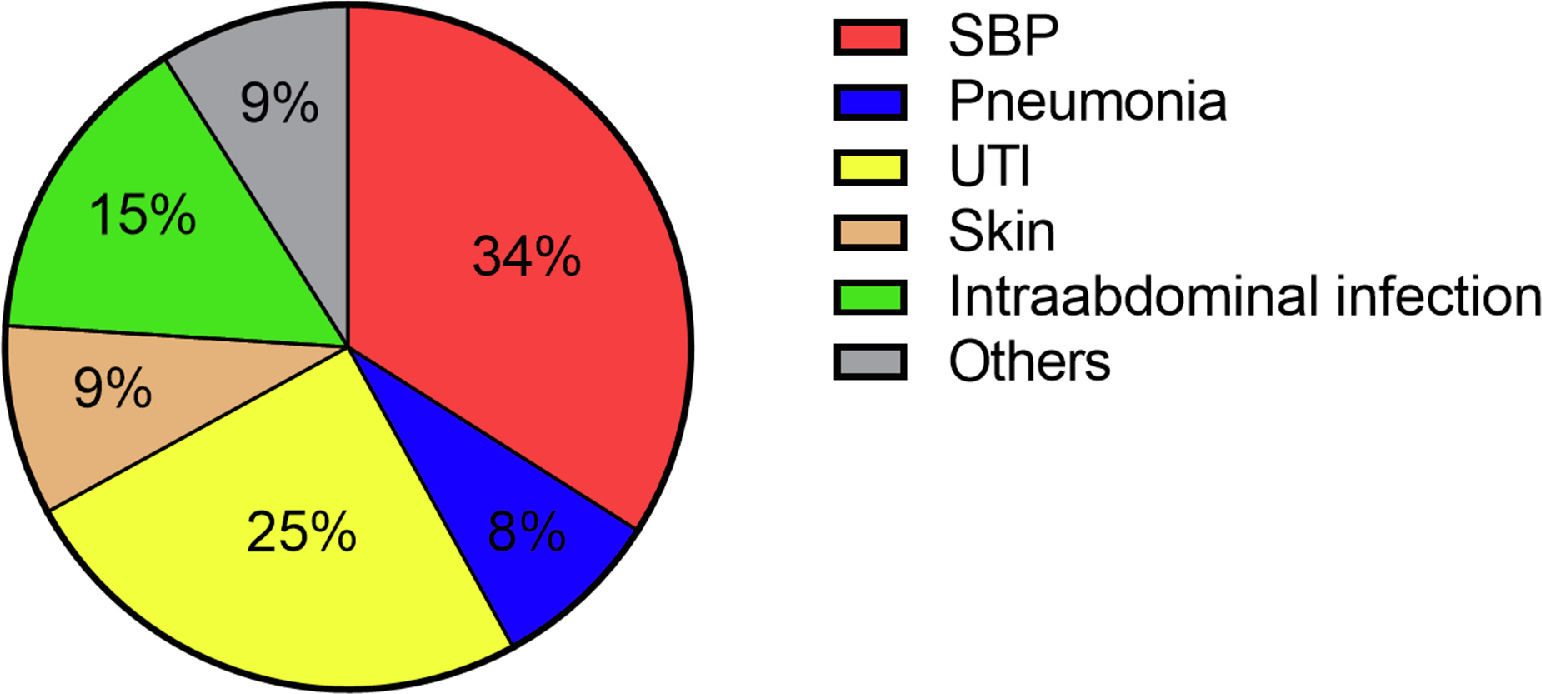
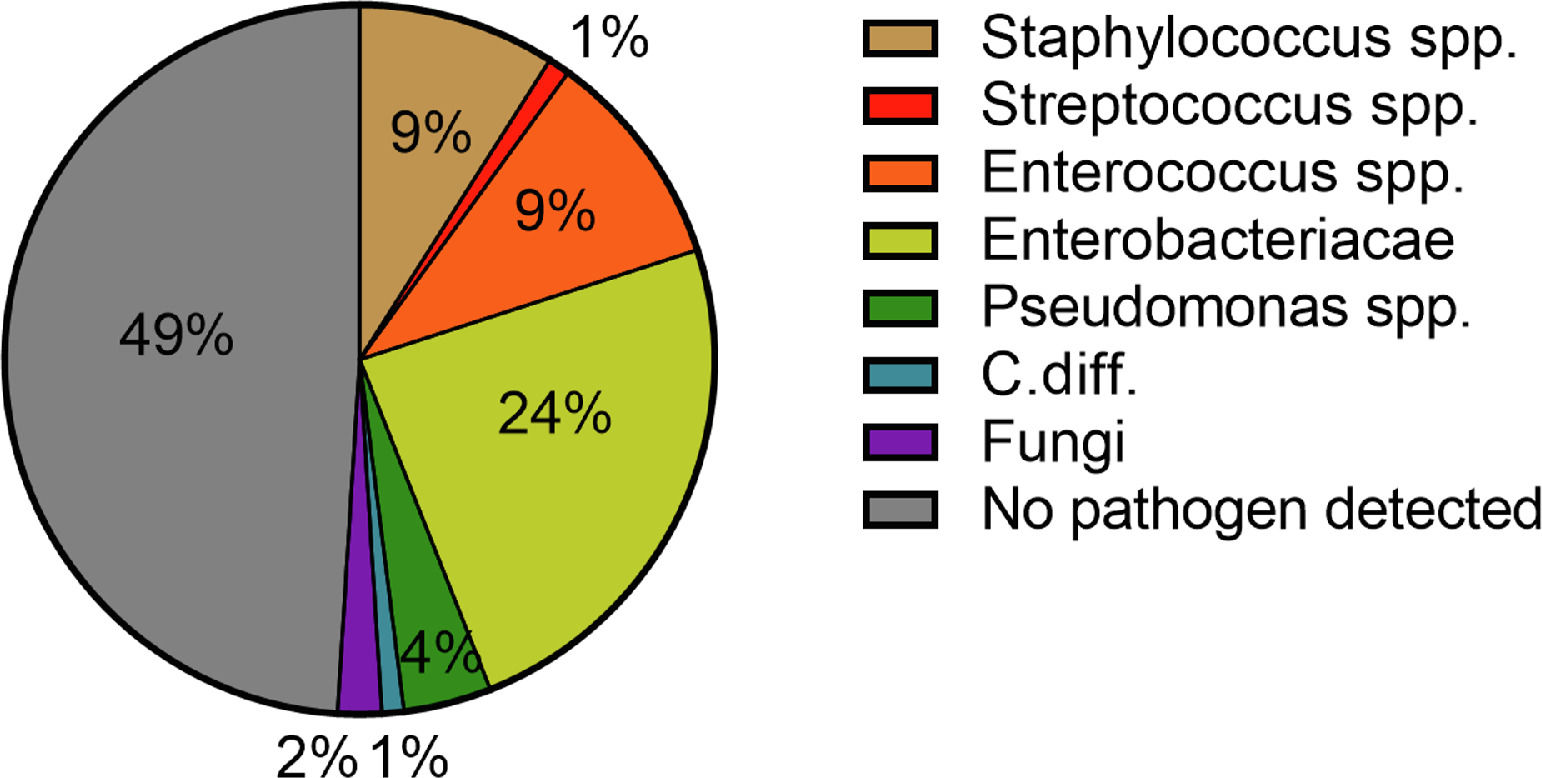
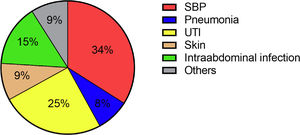
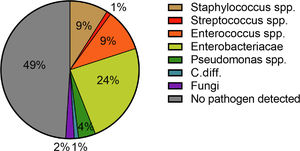
A total of 81 patients (33.9%) suffered from a community-acquired infection. The most common sites were SBP (34.0%), followed by UTI (25.0%) (Fig. 1). In 16/28 (57.1%) patients with SBP and 13/20 (65.0%) patients with UTI a pathogen could be detected. The most common pathogen detected across all infections were Enterobacteriacae (23.8%), followed by Enterococcus (10.0%) and Staphylococcus (8.75%) (Fig. 3). More than half of the patients with a community-acquired infections were initially treated with a calculated 3rd generation cephalosporin antibiotic (55.8%).
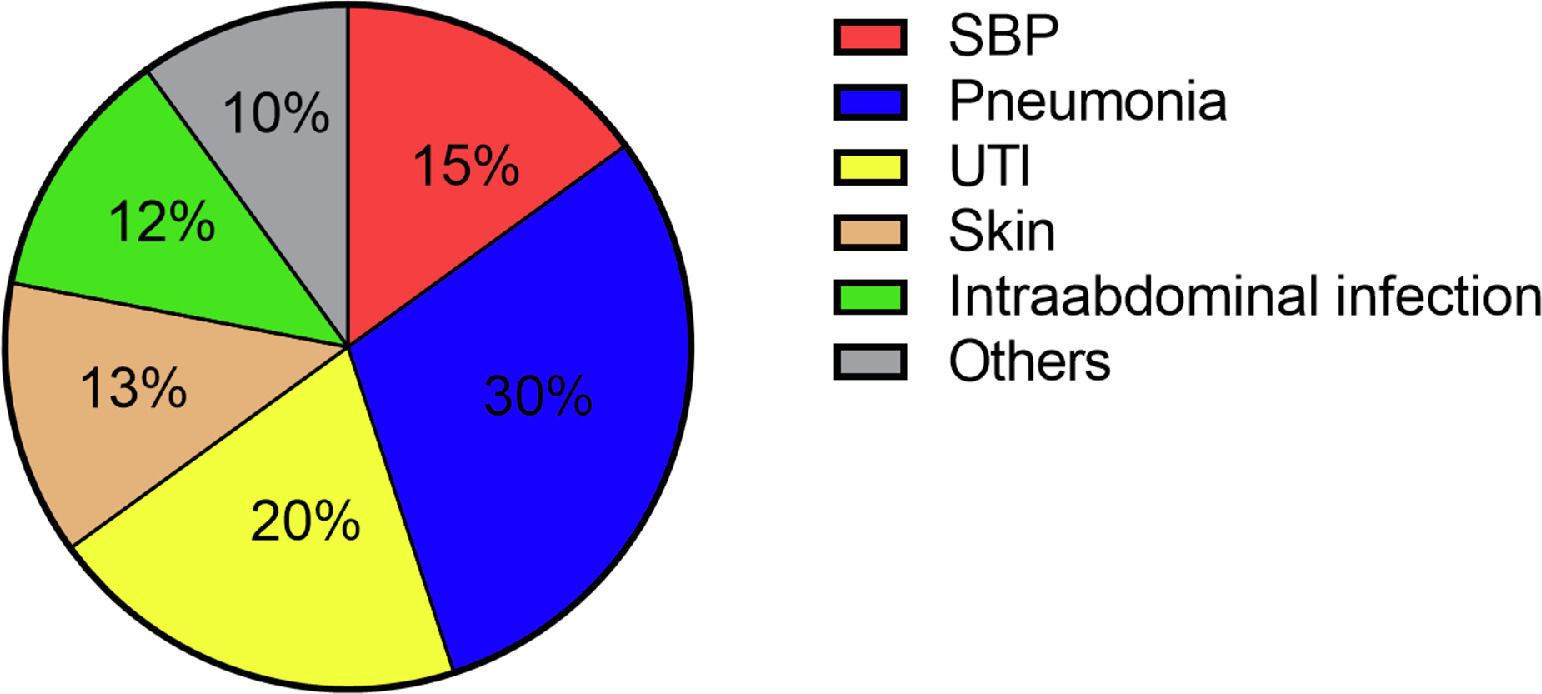
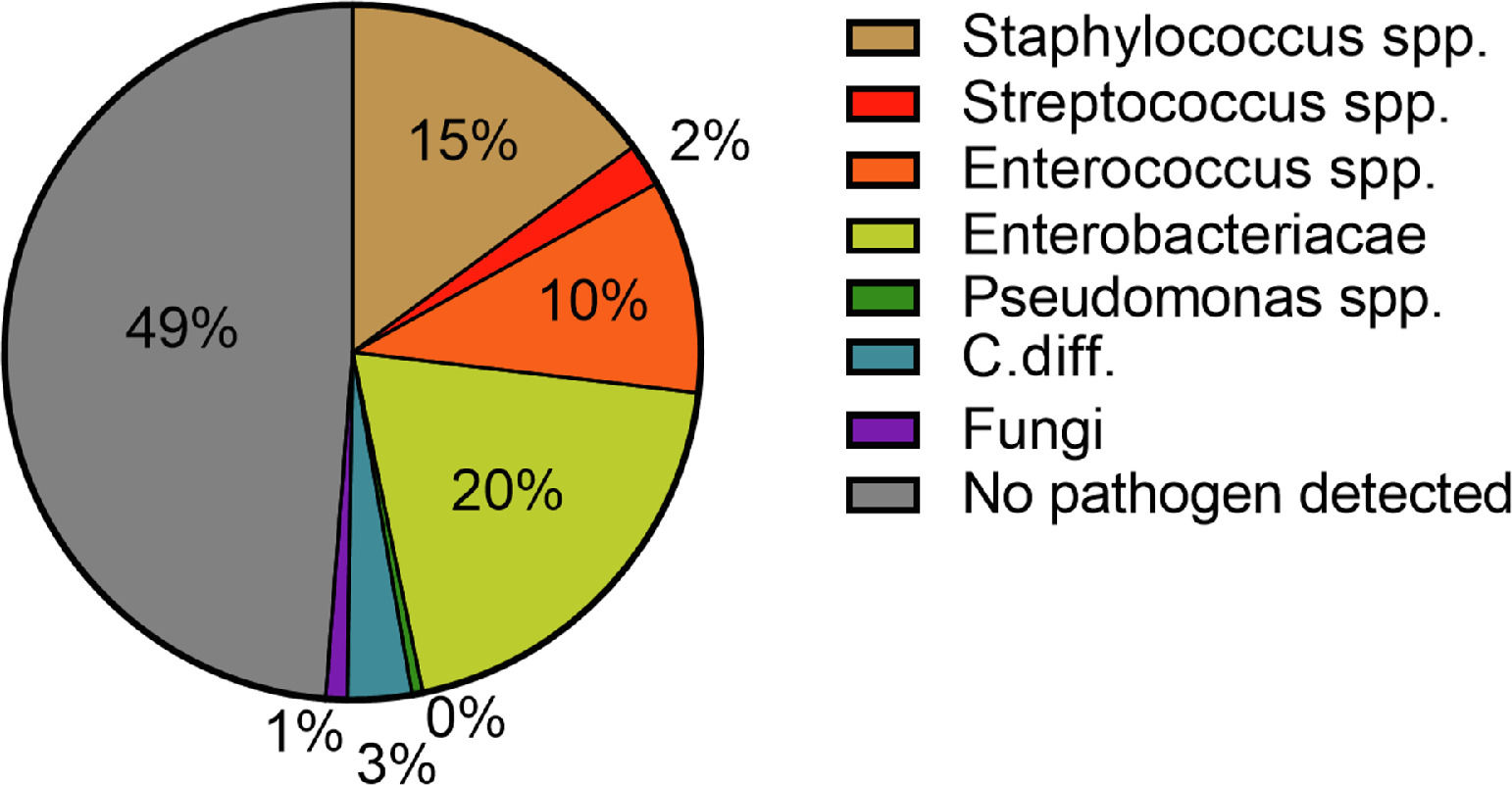
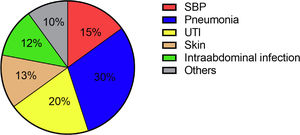
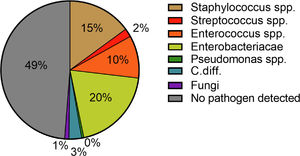
Hospital-acquired infections occurred in 70 patients (29.3%). The most common sites were pneumonia (30.4%), followed by UTI (20.3%) and SBP (14.5%) (Fig. 2). Again, Enterobacteriacae were detected most frequently (20.3%), but in addition, slightly more Gram-positive pathogens were detected in these patients compared to patients with community-acquired infections (26.9%) (Fig. 4). In case of a hospital-acquired infection, antibiotic treatment was most frequently initiated with piperacillin/tazobactam (30.4%). More detailed information on the characteristics of infections is displayed in Table 3.
Characteristics of infections in the study cohort.
Data are expressed as frequencies and percentages.
MDR: multidrug-resistant Gram-negative bacteria; MRSA: Methicillin-resistant Staphylococcus aureus; n: number; SBP: spontaneous bacterial peritonitis; spp: species pluralis; UTI: urinary tract infection; VRE: vancomycin resistant enterococci
MDR bacteria were detected in 7 infected patients (5.8%). MRSA and VRE were only detected once, respectively (Table 3). Of all 239 patients, 127 patients (53.1%) were screened for multidrug-resistant bacteria (colonization) with a swab test. The reason for this is the above-mentioned restrictions on screening, which is why not every patient receives a smear test. 29 (22.8%) patients showed a positive result. Colonization with Gram-negative MDR bacteria was detected most frequently (13.3%), followed by VRE colonization (7.9%).
3.3Impact of bacterial infections on the composite endpoint of death or liver transplantation (mortality)In total, 34 patients died (n = 25, 10.5 %) or received a liver transplantation (n = 9, 3.8 %) during follow-up of 30 days. Given that all patients who had received a liver transplantation had done so because of final hepatic failure, they were treated as complete cases. Of the 25 patients who died, 11 died because of infection, two died because of variceal bleeding, and 12 died because of a combination of complications. The rate of infections during hospital stay was significantly higher in patients who deceased within 30 days compared to patients who survived for more than 30 days (67.6% vs. 48.3%, p < 0.001). The same was true for community-acquired infections (47.1% vs. 31.7%, p < 0.001). Additional characteristics of patients who did and did not decease within 30 days are displayed in Table 4.
Demographics and clinical characteristics of the entire cohort stratified by 30-days mortality at the time of study inclusion.
Data are expressed as medians and interquartile ranges or as frequencies and percentages.
ACLF: acute-on-chronic liver failure; CP: child pugh; CRP: c-reactive protein; HE: hepatic encephalopathy; HRS: hepatorenal syndrome, ICU: intensive care unit; IQR: interquartile range; INR: international normalized ratio; n: number; MELD: model for end-stage liver disease; NAFLD: non-alcoholic fatty liver disease; SBP: spontaneous bacterial peritonitis; wbc: white blood cell; Yr: years
To identify predictors for higher short-term mortality (30 days) in our cohort, we conducted a logistic regression analysis (Table 5). At first, a logistic regression model with stepwise variable selection was conducted including all univariable significant factors (p < 0.05) as displayed in Table 4. Here, a higher MELD score (OR 1.068, 95% CI 1.022 – 1.117, p = 0.004) and the presence of an infection during hospital stay (OR 2.522, 95% CI 1.044 – 6.091, p = 0.040) remained independently associated with higher mortality.
Logistic regression analyses of predictors for the composite endpoint of death/need for liver transplantation within 30 days after hospitalisation.
| Multivariable logistic regression analysis | OR (95% CI) | p-value |
|---|---|---|
| MELDInfection during hospital stay | 1.068 (1.022 – 1.117)2.522 (1.044 – 6.091) | 0.0040.040 |
OR, odds ratio; 95% CI, 95% confidence interval; MELD, model for end-stage liver disease;
Multivariable logistic regression analysis with a stepwise variable selection procedure. Only significant variables are displayed. Not significant were: ACLF, sodium, albumin, haemoglobin, history of ascites, history of hepatic encephalopathy.
To focus on the impact of infections on “true” mortality, we conducted an additional analysis after exclusion of the nine patients who received a liver transplantation. Here, patients with an infection during hospital stay had a higher mortality rate than patients without infections (15.8% vs. 4.3%, p = 0.004). In addition, the mortality rate was numerically higher in patients with community-acquired infections than in patients without community-acquired infections (15.6% vs. 7.2%, p = 0.047) and in patients who developed a hospital-acquired infection than in patients who did not develop a hospital-acquired infection (15.6% vs. 7.9%, p = 0.080).
4DiscussionIn this study, we were able to demonstrate that bacterial infections are common and, along with impaired liver function, the main determinant of short-term mortality in hospitalized patients with liver cirrhosis in Germany. In addition, we may provide a comprehensive overview of the spectrum of infectious pathogens in patients with liver cirrhosis in Germany. In this context, the pathogen pattern shifted from an initial Gram-negative spectrum in community-acquired infections to a more Gram-positive spectrum in hospital-acquired infections. Additionally, we found that MDR bacteria played only a minor role in our cohort, being detected in 5.8% of all infected patients.
It is well-known, that patients with liver cirrhosis have an increased risk of infections [2] and this is validated by our present study. About one in three (33.9%) of the patients presented with community-acquired infections and about one in three (29.3%) developed an infection during their hospital stay. Taken together, every second non-electively hospitalized patient was at risk of infection (50.6%). These numbers are in line with a comparable US-based study [29] and emphasize the fact that a comprehensive diagnostic work-up to detect or exclude infections in hospitalized patients with decompensated cirrhosis is of utmost importance.
Several studies demonstrated the detrimental effect of bacterial infections on short-term prognosis in patients with liver cirrhosis [13,30]. In our cohort, approximately one in three patients with infections did not survive or required a liver transplantation within 30 days of hospitalization. This is explained by a vicious circle including infections, systemic inflammation, and decompensation of cirrhosis frequently resulting in ACLF. In this context, bacteria themselves and their components play an important role in the occurrence of decompensation by triggering an inflammatory cascade [30]. This is supported by the finding that patients with infections had a significantly higher MELD score and the prevalence of ACLF at study inclusion exceeded by far the prevalence of ACLF in patients without infections in our study. This is in line with a recently published German study by Reichert et al. demonstrating that hepatic decompensation in combination with an infection in particular significantly increases mortality, while bacterial infections without decompensation did not result in poorer prognosis [31,32].
In our cohort, the most common site of infection at admission was SBP, followed by UTI. In terms of hospital-acquired infections, we found a relevant change in the sites of infection. Here, most patients suffered from pneumonia, followed by UTI. A hospital-acquired SBP occurred only in a minority of cases. These findings are in line with previously published reports from the western world [33].
Acute decompensation in liver cirrhosis is frequently triggered by infections. However, infections in patients with liver cirrhosis are often clinically less obvious (lower prevalence of fever and lower levels of CRP) than in other patient groups and thus are more difficult to detect. In this context, it is most challenging to detect spontaneous bacteremia caused by portosystemic shunts, which occurs in up to 8-15% of all infections [34]. Therefore, blood cultures should be obtained in every single patient with liver cirrhosis and acute decompensation, even if only mild signs of inflammation are present. In our current study, this workup was insufficiently successful. Peripheral venous blood cultures were only drawn in less than half of all patients (40.1%). Blood cultures from ascites, on the other hand, were taken much more frequently (62.3%).
The pathogen patterns detected in our patients were in line with previously published reports [12,33]. In the majority of cases, Enterobacteriaceae were detected in both community-acquired and hospital-acquired infections (23.8% and 20.3%). Staphylococci spp. and Enterococci spp. were in the second and third place with 8.75% and 14.5% as well as 10.0% and 10.1%, respectively. These findings are consistent with the pathophysiological mechanisms predisposing patients with liver cirrhosis to infections and bacteremia. In this context, the most important factors are liver dysfunction, portal-systemic shunting, CAID, and intestinal bacterial translocation [35]. The later explains above all the increased detection of Gram-negative pathogens [35]. The detection-rate of Gram-positive pathogens can be explained by the increase in rates of skin infections in patients with hospital-acquired infections. The main triggers here were intravenous lines. Here, an increase of Staphylococcus spp. could be detected (42.9% (n =3/7) to 90.0% (n = 9/10)). Despite the significant increase in pneumonia, pathogen detection was often not possible. The pathogen was detected in only two out of six cases and in four out of 21 cases of nosocomial pneumonia.
In the context of pathogen patterns in patients with cirrhosis, infections with MDR pathogens are known to be rising worldwide [12,29,36]. Three previous studies including patients from tertiary care centers found a prevalence of infections with MDR bacteria ranging from 23% up to 47% [12,29,36]. Another German study conducted by Hillert et al. found a prevalence of 19.2% of MDR bacteria in hospitalized patients with ascites [37]. In contrast, the prevalence of MDR bacteria in patients with infections was 5.8% in our study. When interpreting our findings in the context of these aforementioned studies, it is a strength of our study that we provide a comprehensive picture of detected bacterial pathogens in an unselected cohort of hospitalized patients with liver cirrhosis, while e.g. the study by Hillert et al. only investigated patients with ascites [37]. Although the large difference in the prevalence of MDR bacteria is somewhat surprising, these results highlight the heterogeneity of prevalence rates even among tertiary care centers in Germany. In addition, Hillert et al. also reported an overall reduction in MDR bacteria when comparing time frames before and after 2015 (34.0% vs. 19.5%). This underlines not only the local but also the temporal heterogeneity of pathogen spectra. Therefore, prevalence rates of MDR bacteria should be closely monitored in centers with a focus on managing liver cirrhosis to adjust recommendations regarding calculated antibiotic treatment regimens in these patients. Currently, the EASL recommends piperacillin/tazobactam instead of 3rd generation cephalosporins, if the pathogen pattern in the respective region corresponds to a high risk of MDR bacteria [6]. Based on our current findings, it seems reasonable to stick with 3rd generation cephalosporines in our center given the low prevalence of MDR bacteria.
Our study has several limitations that have to be acknowledged. First, our study design is observational, and the assessment of infections was done according to the local standard of care. Therefore, our findings especially regarding the prevalence of detected pathogens have to be interpreted in the context of the design. Additionally, it has to be acknowledged that in some patients microbial diagnostics were missing. This is likely explained by unintentional omission in daily routine. Our study reflects the real-life scenario at a tertiary care center in Germany, and we therefore believe that this finding should further raise awareness of the need for consistent bacterial diagnosis in hospitalized patients with liver cirrhosis. Second, this study was conducted at a tertiary care center with a special focus on liver transplantation. This may result in selection bias and our findings may not be representative for hospitals without a liver transplantation program. Last, the frequency of outcome events in our study was comparably small. Therefore, the multivariable analysis of our study should be interpreted with caution. Additionally, due to the low number of events we are unable to conduct multivariable analysis with the outcome mortality.
5ConclusionsIn conclusion, this study demonstrates that bacterial infections are common in hospitalized patients with liver cirrhosis in Germany and are, along with impaired liver function, a major determinant of short-term mortality. Additionally, we found that pathogen spectra changed from a Gram-negative spectrum in community-acquired infections to a more Gram-positive spectrum in hospital-acquired infections with a low frequency of MDR bacteria of 5.8%. Our data highlight the importance of regional differences in MDR bacteria and may guide physicians' decision-making regarding calculated antibiotic treatment.
Author contributionsPerformed research: W.M.K., E.I., V.K., C.L. Contributed to acquisition of data: W.M.K., L.K., M.H., E.I., V.K., M.M., J.M.S., M.F.S., M.A.W., M.N., C.L. Designed the experiments and analyzed the data: W.M.K., M.A.W., C.L. Contributed reagents/materials/analysis tools: P.R.G., M.A.W., C.L., S.J.G., W.M.K. Wrote the paper: W.M.K., C.L. Statistical analysis: W.M.K, C.L. All authors approved the final version of the manuscript and the authorship list.
Data availability statementThe datasets generated and analysed during the current study are not publicly available due ethical restrictions but are available from the corresponding author on reasonable request.
Declaration of interestNone