Algorithms can have several purposes in the clinical practice. There are different scales for causality imputation in DILI (Drug-Induced Liver Injury), but the applicability and validity of these for the HILI (Herb-Induced Liver Injury) evaluation is questionable for some scales. The purpose of the study was to determine the clinical and demographic profile of the patients with HILI, and the main algorithmic scales used in its causality assessment. The methodology was a systematic review of articles in English, Spanish, or Portuguese language, from 1979 to 2019, involving humans, with descriptors related to HILI. Qualitative and quantitative statistical analysis were performed. As a result, from a total of 60 articles, 203 HILI reports were selected: 59.9% were women, similar with other studies, and the average age was 45.8 years. Jaundice was the most frequent symptom and regarding the type of lesion, the hepatocellular was the most frequent. In regard to HILI severity, 3.0% were severe and 7.6% were fatal or required liver transplantation. In 72.3% of the cases, the most used algorithm was RUCAM (Roussel Uclaf Causality Assessment Method). The conclusion of the study is that RUCAM was the most used algorithm for causality assessment in HILI. The patients were predominantly female, jaundice was the main symptom, and HILI is reversible in the majority of cases.
DILI groups include drug-induced and xenobiotic-induced hepatotoxicity [1]. The histopathology is rich and may contain several findings [2,3]. The term HILI encompasses cases of herb-induced liver injury [4]. According to WHO, 85% of the developing countries population uses these products in their primary health care [5]. Some studies on the herbs and herbal products most consumed in the West and possibly associated with liver injury indicate that green tea is the main product associated with HILI, while in the East the natural products most associated with HILI are called Traditional Chinese Medicine (TCM) [6]. The incidence of liver injury associated with herbal products is uncertain, due to the scarcity of epidemiological studies related to the subject.
Algorithms can have several purposes in the clinical practice. The most common approach for diagnosis and treatment, is a “checklist” to define conducts. An example of an algorithm used in imputation of causality in HILI is the RUCAM (Roussel Uclaf Causality Assessment Method) score, the algorithmic tool most used in current clinical practice for the evaluation of hepatotoxicity [7,8]. There are several algorithms used to evaluate HILI, however, there is still no reproducible and practical method to predict, diagnose and evaluate the risk of this kind of hepatotoxicity [9].
The aim of this study is to determine the clinical and demographic profile of the population that had HILI and the main algorithmic scales used in its causality assessment.
2Material and methodsThis is a systematic literature review conducted in the Medline, Scopus, Lilacs and Web of Science databases, the MESH descriptors were: chemically-induced liver toxicity; drug-induced acute liver injury; drug-induced liver disease; drug-induced liver injury; dietary supplement; food supplementations; nutraceuticals; herbal products; herbal therapy; Chinese drugs, plant; plant extracts; medicinal plants; pharmaceutical plants; healing plants; medicinal herbs. Boolean connectors used were “AND” and “OR”. The articles in which fulltexts were not available were requested by an e-mail to the author to recover the original work.
The included articles were case reports and series cases, with full text available, published from 1979 to 2019 and involving humans. The selected articles had their titles, abstracts and methodologies read. Studies that did not use any algorithm for HILI evaluation, that did not describe HILI or that did not provide data on the cases were excluded.
To characterize the cases as HILI, the criteria by Danan et al. [10–12] were used: alanine aminotransferase (ALT) ≥ 5 x the upper limit of normal (ULN) or alkaline phosphatase (ALP) ≥ 2 x ULN. Imaging during the episode and / or histological summary, liver biopsy, serology of hepatitis caused by hepatotropic virus or other liver disease based on clinical characteristics and laboratory tests were also associated. The type of the liver injury was based on R values (ALT / ULN) / (ALP / ULN).
The HILI severity were classified according to International DILI Expert Working Group as: mild (high alanine aminotransferase / alkaline phosphatase (ALT / ALP) activities meeting criteria for HILI and bilirubin concentration <2 × upper limit of normal (ULN), moderate (high ALT / ALP activities meeting criteria for HILI but bilirubin concentration ≥2 x ULN, or symptomatic hepatitis), severe (high ALT / ALP activities meeting criteria for HILI, bilirubin concentration ≥2 x ULN and any of the following: international normalized ratio ≥ 1.5; ascites and / or encephalopathy, disease duration <26 weeks and absence of underlying cirrhosis; other organ failure (considered due to HILI)) or fatal (death or transplantation caused by HILI, based on HILI severity rating).
Independent researchers conducted the data extraction. The following data were retrieved and analyzed from each article identified in the literature search: country source of the report, demographics, suspected herbal medicine, type of liver injury, biochemical parameters, histological findings, and outcome of the reaction.
Data analysis was performed qualitatively and quantitatively, using the Statistical Package for the Social Sciences (SPSS) version 21.0. Pearson's Chi Square Test was performed for categorical variables and Student's t-test for average comparisons. Statistical significance was considered p < 0.05.
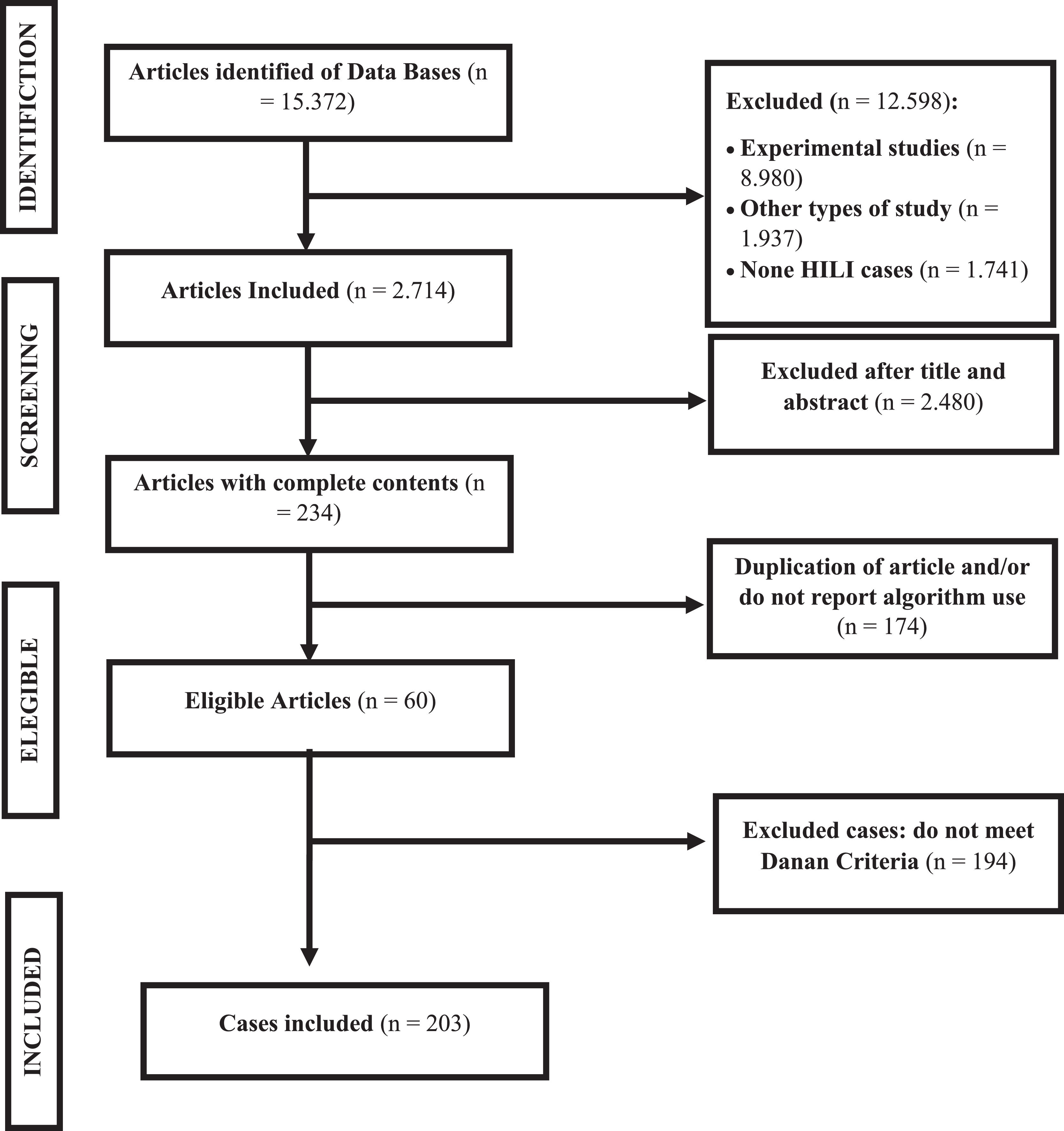
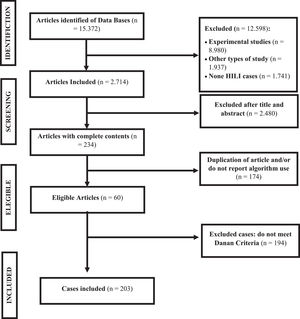
3ResultsA total of 60 articles were found that described cases of HILI and reported the use of at least one algorithm; of these, 203 cases were in line with Danan criteria and were included in this study (Fig. 1). The articles were from European (46.5%), Asians (31.2%), Americans (10.9%), Africans (10.9%) and Oceania (0.5%) publications. Among these, 59.9% were women, and age ranged from 1 to 82 years, with an average of 45.8 years. The percentage of elderly individuals (over 60 years) was 19.9%.
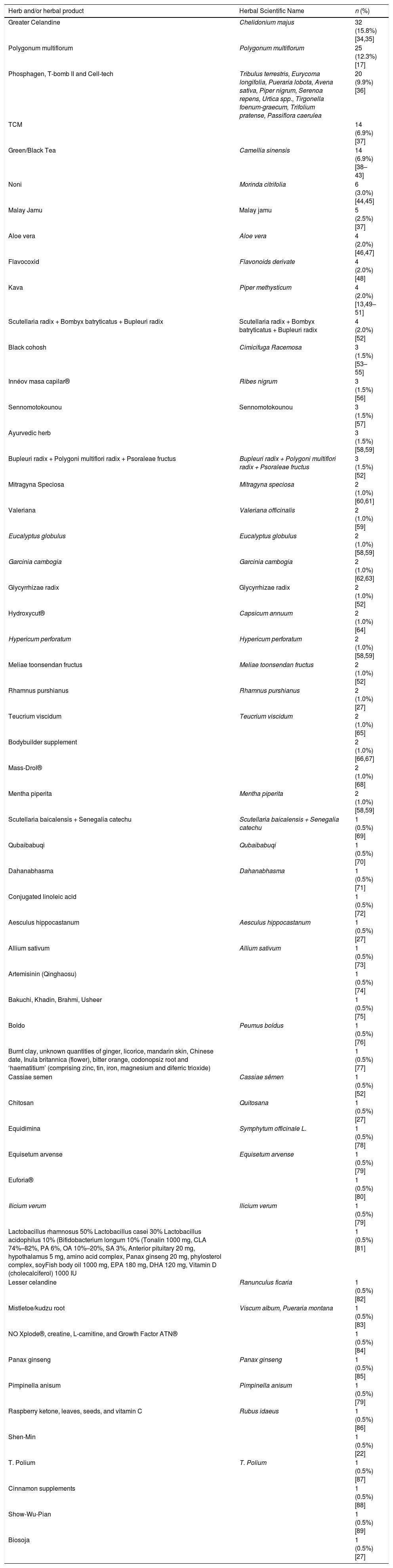
The main natural and herbal products used were: Chelidonium majus (15.8%) [25,26], Polygonum multiflorum (12.3%) [27], Phosphagen, T-bomb II and Cell-tech (9.9%) [28], TCM (6.9%) [29] and green/black tea (6.9%) [30–36] (Table 2). About 74% of patients reported using only one hepatotoxic herbal product. The most frequent indications for use were to promote general/gastrointestinal well-being (25.2%), musculoskeletal pain (19.3%), skin and phanises lesions (4.5%), central nervous system-related complaints (4.0%). The hormonal disease/menopausal symptoms and systemic infections were 1.5% each. Sexual stimulator, antiparasitic, respiratory problems, cardiovascular disorders, diseases of the blood and hematopoietic tissue and immune system regulators were 0.5% each. About 4.0% of the cases had other non-standard indications for use; 37% of the cases omitted the indication of use.
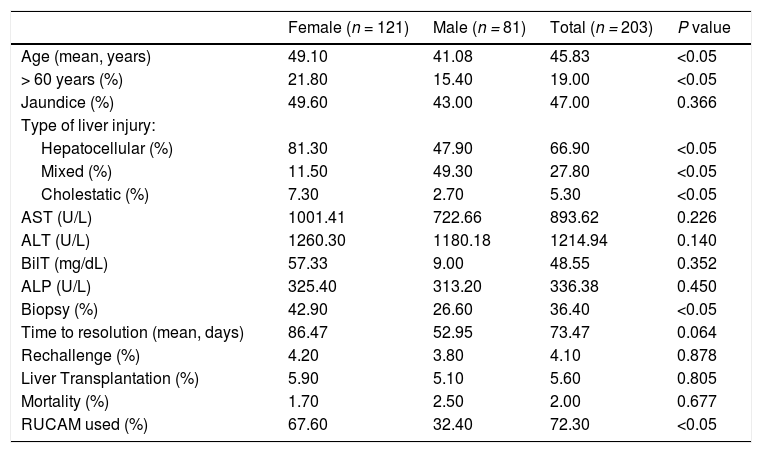
The main symptom presented by HILI patients was jaundice (47%). Regarding the biochemical profile, AST = 893.62 U/L; ALT = 1.214.94 U/L; BT = 48.55 mg/dL; ALP = 336.38 U/L. There were no significant differences between the laboratory findings of the female and male cohort (Table 1).
Clinical and demographics characteristics of HILI cases published in literature from 1979 to 2019.
ALT: Aspartate Aminotransferase.; AST: Alanine Aminotransferase; BT: Total Bilirubin; ALP: Alkaline phosphatase; RUCAM: Roussel Uclaf Causality Assessment Method.
Principal Herbs and herbal products associated with HILI cases.
| Herb and/or herbal product | Herbal Scientific Name | n (%) |
|---|---|---|
| Greater Celandine | Chelidonium majus | 32 (15.8%) [34,35] |
| Polygonum multiflorum | Polygonum multiflorum | 25 (12.3%) [17] |
| Phosphagen, T-bomb II and Cell-tech | Tribulus terrestris, Eurycoma longifolia, Pueraria lobota, Avena sativa, Piper nigrum, Serenoa repens, Urtica spp., Tirgonella foenum-graecum, Trifolium pratense, Passiflora caerulea | 20 (9.9%) [36] |
| TCM | 14 (6.9%) [37] | |
| Green/Black Tea | Camellia sinensis | 14 (6.9%) [38–43] |
| Noni | Morinda citrifolia | 6 (3.0%) [44,45] |
| Malay Jamu | Malay jamu | 5 (2.5%) [37] |
| Aloe vera | Aloe vera | 4 (2.0%) [46,47] |
| Flavocoxid | Flavonoids derivate | 4 (2.0%) [48] |
| Kava | Piper methysticum | 4 (2.0%) [13,49–51] |
| Scutellaria radix + Bombyx batryticatus + Bupleuri radix | Scutellaria radix + Bombyx batryticatus + Bupleuri radix | 4 (2.0%) [52] |
| Black cohosh | Cimicifuga Racemosa | 3 (1.5%) [53–55] |
| Innéov masa capilar® | Ribes nigrum | 3 (1.5%) [56] |
| Sennomotokounou | Sennomotokounou | 3 (1.5%) [57] |
| Ayurvedic herb | 3 (1.5%) [58,59] | |
| Bupleuri radix + Polygoni multiflori radix + Psoraleae fructus | Bupleuri radix + Polygoni multiflori radix + Psoraleae fructus | 3 (1.5%) [52] |
| Mitragyna Speciosa | Mitragyna speciosa | 2 (1.0%) [60,61] |
| Valeriana | Valeriana officinalis | 2 (1.0%) [59] |
| Eucalyptus globulus | Eucalyptus globulus | 2 (1.0%) [58,59] |
| Garcinia cambogia | Garcinia cambogia | 2 (1.0%) [62,63] |
| Glycyrrhizae radix | Glycyrrhizae radix | 2 (1.0%) [52] |
| Hydroxycut® | Capsicum annuum | 2 (1.0%) [64] |
| Hypericum perforatum | Hypericum perforatum | 2 (1.0%) [58,59] |
| Meliae toonsendan fructus | Meliae toonsendan fructus | 2 (1.0%) [52] |
| Rhamnus purshianus | Rhamnus purshianus | 2 (1.0%) [27] |
| Teucrium viscidum | Teucrium viscidum | 2 (1.0%) [65] |
| Bodybuilder supplement | 2 (1.0%) [66,67] | |
| Mass-Drol® | 2 (1.0%) [68] | |
| Mentha piperita | Mentha piperita | 2 (1.0%) [58,59] |
| Scutellaria baicalensis + Senegalia catechu | Scutellaria baicalensis + Senegalia catechu | 1 (0.5%) [69] |
| Qubaibabuqi | Qubaibabuqi | 1 (0.5%) [70] |
| Dahanabhasma | Dahanabhasma | 1 (0.5%) [71] |
| Conjugated linoleic acid | 1 (0.5%) [72] | |
| Aesculus hippocastanum | Aesculus hippocastanum | 1 (0.5%) [27] |
| Allium sativum | Allium sativum | 1 (0.5%) [73] |
| Artemisinin (Qinghaosu) | 1 (0.5%) [74] | |
| Bakuchi, Khadin, Brahmi, Usheer | 1 (0.5%) [75] | |
| Boldo | Peumus boldus | 1 (0.5%) [76] |
| Burnt clay, unknown quantities of ginger, licorice, mandarin skin, Chinese date, Inula britannica (flower), bitter orange, codonopsiz root and ‘haematitium’ (comprising zinc, tin, iron, magnesium and diferric trioxide) | 1 (0.5%) [77] | |
| Cassiae semen | Cassiae sêmen | 1 (0.5%) [52] |
| Chitosan | Quitosana | 1 (0.5%) [27] |
| Equidimina | Symphytum officinale L. | 1 (0.5%) [78] |
| Equisetum arvense | Equisetum arvense | 1 (0.5%) [79] |
| Euforia® | 1 (0.5%) [80] | |
| Ilicium verum | llicium verum | 1 (0.5%) [79] |
| Lactobacillus rhamnosus 50% Lactobacillus casei 30% Lactobacillus acidophilus 10% (Bifidobacterium longum 10% (Tonalin 1000 mg, CLA 74%–82%, PA 6%, OA 10%–20%, SA 3%, Anterior pituitary 20 mg, hypothalamus 5 mg, amino acid complex, Panax ginseng 20 mg, phylosterol complex, soyFish body oil 1000 mg, EPA 180 mg, DHA 120 mg, Vitamin D (cholecalciferol) 1000 IU | 1 (0.5%) [81] | |
| Lesser celandine | Ranunculus ficaria | 1 (0.5%) [82] |
| Mistletoe/kudzu root | Viscum album, Pueraria montana | 1 (0.5%) [83] |
| NO Xplode®, creatine, L-carnitine, and Growth Factor ATN® | 1 (0.5%) [84] | |
| Panax ginseng | Panax ginseng | 1 (0.5%) [85] |
| Pimpinella anisum | Pimpinella anisum | 1 (0.5%) [79] |
| Raspberry ketone, leaves, seeds, and vitamin C | Rubus idaeus | 1 (0.5%) [86] |
| Shen-Min | 1 (0.5%) [22] | |
| T. Polium | T. Polium | 1 (0.5%) [87] |
| Cinnamon supplements | 1 (0.5%) [88] | |
| Show-Wu-Pian | 1 (0.5%) [89] | |
| Biosoja | 1 (0.5%) [27] |
Regarding the type of liver injury, hepatocellular liver damage (66.9%) was the most frequent. The majority of patients (94.5%) were completely recovered from the liver injury; the average time to resolution was 73 days, with a median of 40 days and standard deviation equal to 129.9 days. Approximately 4.1% of the individuals were re-exposed to the culprit of HILI (Table 1).
The application of Student's t test for mean age revealed a statistically significant higher incidence of women over 60 years of age. From the analysis of the categorical variables, an association between hepatocellular lesion profile and female gender was observed. In addition, the female population had more indications for biopsy.
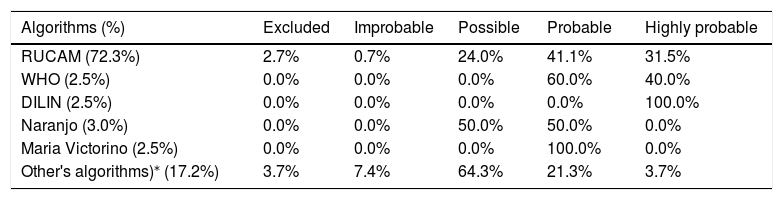
About severity, 9.8% were mild, 79.6% were classified as moderate, 3.0% were severe and 7.6% were fatal or required liver transplantation. Of those who died or required liver transplantation, only 36.4% underwent biopsy (Table 1). The most used algorithm for the HILI causality assessment in the collected cases was RUCAM, applied in about 72.3% of patients, predominantly among women (Table 3).
Algorithms used in the assessment of HILI causality in published cases from 1979 to 2019.
| Algorithms (%) | Excluded | Improbable | Possible | Probable | Highly probable |
|---|---|---|---|---|---|
| RUCAM (72.3%) | 2.7% | 0.7% | 24.0% | 41.1% | 31.5% |
| WHO (2.5%) | 0.0% | 0.0% | 0.0% | 60.0% | 40.0% |
| DILIN (2.5%) | 0.0% | 0.0% | 0.0% | 0.0% | 100.0% |
| Naranjo (3.0%) | 0.0% | 0.0% | 50.0% | 50.0% | 0.0% |
| Maria Victorino (2.5%) | 0.0% | 0.0% | 0.0% | 100.0% | 0.0% |
| Other's algorithms)⁎ (17.2%) | 3.7% | 7.4% | 64.3% | 21.3% | 3.7% |
RUCAM: Roussel Uclaf Causality Assessment Method; WHO: World Health Organization; DILIN: Drug Induced Liver Injury Network.
Other algorithms used for causal attribution: Clinical Diagnostic Scale (CDS); European Medicines Agency (EMEA); Drug Interaction Probability Scale (DIPS); Drug Commission of the German Medical Association (DCGMA); Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM); Federal Institute for Drugs and Medicinal Products (FIDMP).
In this review, 60 articles and 203 cases with HILI were found. In comparison, other review studies have identified 14.029 cases of HILI [13]. This higher number can be justified by the criteria established for the selection of the cases. Using similar criteria, Becker in his review of Brazil's cases, identified only three cases of HILI, published in the literature. As for the origin of the publications in this review, data is similar, most of them were outside Europe and Asia, in which the countries with the highest number of published cases were China, Korea and Germany [13,14].
In the present study, 59.9% of the population was female, with an average age of 45.8 ± 16.2 years and a profile of predominantly hepatocellular liver injury. The clinical characteristics in HILI cases retrieved in our review was compared to those of the Spanish DILI Registry [15], US DILIN [16] and Teschke et al. [13]. According to Jung et al [17], 69.8% of the population with HILI was female, with an average age of 48.7 ± 11.3 years, and a predominantly hepatocellular lesion profile [15].
Frenzel et al. [18] revealed that green tea and black tea are among the most consumed herbal medicines in the world. In 2019 the main products associated with HILI were Greater celadine (20.8%), Teucrium racemosum (18.9%) and Teucrium polium (5.7%) [19]. Another study showed that Polygonum multiflorum was the herbal product most associated with HILI (39.2%) [20]. Similarly, among the main herbal products found in this review were: Greater Celadine (15.8%) and Polygonum multiflorum (12.3%), which matches the data published.
The mechanism for the etiology of HILI is understood. According to published studies, more than 20 different kinds of alkaloids can be found in Greater celandine, including chelerythrine, sanguinarine, berberine, chelidonine, and coptisine [21]. Cases of hepatotoxicity due to Polygonum multiflorum have been reported in China, Australia, and Italy [22]. The anthraquinones, an active ingredient of Polygonum multiflorum is considered to play a role as the potential cause of hepatotoxicity, but previous reports were nonconclusive [23–25].
As in other studies from the USA and Europe, [16] the main prescription reason for herbal products used is weight loss. According to Santos et al. [26], the main indication for the intake of natural and herbal products was weight loss (52%), followed by: relieving the symptoms of digestive disorders, constipation, hair loss, arthralgia, diabetic neuropathy, anxiety and maintaining general health. In the present study, the symptoms of digestive disorders and the musculoskeletal pain treatment were the main indications for the use of the products, according to the data found in the literature.
The most found symptom in the population was jaundice (47%), this is same with the Spanish Group [15] and Frenzel et al. [18] but the incidence higher than that in the present study. Regarding biomarkers, Lin NH et al. [19] showed that the average ALT values of HILI-related liver lesions was 1205 U/L, while the average ALP was 253.2 U/L. Compared this review, ALT and ALP were 1214 U/L and 336 U/L, respectively. The superior laboratory results found in the present study can be justified using the Danan criteria for the selection of studies. Therefore, HILI suspected cases with minimal or transitory ALT/AF elevations were excluded from the study.
In the current HILI case series, 94.5% of the patients were complete recovery. Drug-Induced Liver Injury Network (DILIN) study and García-Cortés M et al. [16,27] previously reported that HILI patients required more medical interventions and were associated with a higher risk of a severe outcome compared to DILI [23]. The time to resolution of HILI was similar with Lin et al. [19] the average was 78 days, while the time estimated in this study was 73 days. Approximately 4.1% of the individuals were re-exposed to the culprit HILI agent; according to Santos et al. [26], most patients tend to hide this information from the doctor, and it is not, in most studies, a reliable data. Regarding the degree of HILI severity, the majority was considered moderate (79.6%), according to International DILI Expert Working Group criteria. A study [28] involving 488 cases showed that HILI mortality was 4.1%. Within the study population, 36.4% underwent biopsy; 5.6% required liver transplantation and only 2.0% died. The lack of follow-up greater than 6 months by most reports and case series may have underestimated the percentage of patients who died within the sample.
Danan et al. [29,30] demonstrated that currently, RUCAM is the gold standard in the DILI/HILI assessment, with the most comprehensive validated scale available. Teschke et al. [31] revealed that 46,266 DILI cases worldwide were evaluated by RUCAM between 2014 and 2019, being the most used tool in world clinical practice. In 2019, 11,609 HILI incidents were reported by the RUCAM scale, a number much higher than that reported in previous years (about 100-1000 cases). The most used algorithm for the HILI quantification, in the collected cases, was RUCAM, applied in about 72.3% of patients. The results found in the group were: 24.0% possible, 41.1% probable and 31.5% highly probable. The WHO, Naranjo and Maria Victorino scales were the most used in the group of patients in which the RUCAM was not applied, being present mainly in the reports and series of older cases, period prior to the publication of studies that proved the good RUCAM performance in large populations and stimulating its use in the clinical management of HILI patients.
According to the recent publications on causality assessment of HILI, RUCAM is the preferred algorithm for the imputation of causal nexus in cases of liver injury and herbal products. This demonstrates the acceptance of this algorithm among experts [13,32].
In the present study, most of the selected HILI cases used the RUCAM, although it was not developed for this purpose, the validation of liver injuries by natural products requires a more precise investigation, on the suspected agent, as: information on phytochemistry that can be correlated to liver damage and the content of these active compounds. Another aspect is the presence of microbiological and physicochemical contaminants, which would require better detailing of the cases to exclude alternative causes. According to Teschke [32], HILI is a complex condition where it needs a more precise approach, especially for the time of events, and the exclusion of alternative causes is necessary.
In regard to the grade found after using RUCAM in the studies, in this review, most cases were classified as possible or probable. The evaluation of retrospective cases by RUCAM, leads to low scores and lower causality classifications, due to low or negative scores, which shows weak robustness in case investigation. Randomized controlled trials are needed to establish a good benefit on the balance of risk for safe use of natural products [32].
However, Danan et al. [29,30], and Becker et al. [33] argued in their studies that RUCAM should continue to be the gold standard in the DILI/HILI evaluation and reinforced that its use to determine causality can be done retrospectively but RUCAM should better be used prospectively to collect complete data required for high causality gradings. Therefore, early application should be stimulated for the patients care with suspected HILI.
5ConclusionThe study showed that the population affected by HILI is predominantly female, with an average age of 45.8 years and a profile of hepatocellular liver injury. The main symptom presented was jaundice. RUCAM scale was the most applied in the cases of our study. There are no studies to date that compare the efficiency and superiority of scales in the DILI/HILI evaluation and quantification.AbbreviationsALT Alanine Aminotransferase Alkaline Phosphatase Aspartate Aminotransferase Bundesinstitut für Arzneimittel und Medizinprodukte Bilirubins Total Clinical Diagnostic Scale Council for International Organizations of Medical Sciences Drug Commission of the German Medical Association Drug induced liver injury Drug Induced Liver Injury Network Drug Interaction Probability Scale European Medicines Agency Federal Institute for Drugs and Medicinal Products Gamma Glutamyl Transferase Herb Induced Liver Injury Roussel Uclaf Causality Assessment Method Statistical Package for the Social Sciences Traditional Chinese Medicine Upper Limit of Normality World Health Organization
The present study has been supported by grants of the Maria Emilia Pedreira Freire de Carvalho Foundation, Brazil.Permission to reproduce material from other sources: None.Pedro Felipe Soares: Collected, analyzed data, and wrote manuscript.Maria Tereza Calchi Fanti Fernandes: Collected data.Andréia de Santana Souza: Collected data.Caio Medina Lopes: Collected data.Darjore Amorim Carvalho dos Santos: Collected data.Diogo Pereira Rodrigues Oliveira: Collected data.Marcela Gottschald Pereira: Collected data.Nilia Maria De Brito Lima Prado: Designed the study, revised the manuscript.Gecynalda Soares da Silva Gomes: Analysis Statistics and interpretation of data.Genário Santos: Designed the study, revised the manuscript.Raymundo Paraná: Designed the study, revised the manuscript.