Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal recessive cholestatic diseases of childhood and represents the main indication for liver transplantation at this age; PFIC2 involves ABCB11 gene, that encodes the ATP-dependent canalicular bile salt export pump (BSEP). Benign intrahepatic cholestasis (BRIC) identifies a group of diseases involving the same genes and characterized by intermittent attacks of cholestasis with no progression to liver cirrhosis. Diagnosis with standard sequencing techniques is expensive and available only at a few tertiary centers. We report the application of next generation sequencing (NGS) in the diagnosis of the familial intrahepatic cholestasis with a parallel sequencing of three causative genes. We identified the molecular defects in ABCB11 gene in two different probands who developed a severe cholestatic disease of unknown origin. In the first patient a compound heterozygosity for the novel frameshift mutation p.Ser1100GlnfsX38 and the missense variant p.Glu135Lys was detected. In the second patient, triggered by contraceptive therapy, we identified homozygosity for a novel mis-sense variant p.Ala523Gly. In conclusion, these mutations seem to have a late onset and a less aggressive clinical impact, acting as an intermediate form between BRIC and PFIC.
Progressive Familial Intrahepatic Cholestasis (PFIC) is a heterogeneous group of autosomal recessive disorders of childhood presenting with intrahepatic cholestasis and characterized by defects in biliary proteins that are involved in the synthesis and transport of bile.1
These diseases, usually lead within the first decade of life to portal hypertension, liver failure, liver cancer and extra-hepatic manifestations often requiring liver transplantation.
Up to now, three different types have been identified: PFIC1, PFIC2 and PFIC3 caused by mutations in ATP8B1, ABCB11 and ABCB4 genes respectively.
PFIC2, also known as BSEP deficiency, involves ABCB11 gene that encodes the bile salt export pump (BSEP), a liver-specific adenosine triphosphate (ATP)-binding cassette transporter, that mediates the excretion of bile monovalent salts.2
There are two different phenotypes, according to the activity of the enzyme serum gamma-glutamil-transpeptidase (gGT): PFIC1 and PFIC2 are characterized by low or normal gGT and elevation of alkaline phosphatase (AP) while the PFIC3 presents elevation of both enzymes. Patients usually present in early childhood with cholestasis, recurrent episodes of jaundice, often associated with uncontrollable pruritus.
Benign Intrahepatic Cholestasis (BRIC) type 2 is a rare disorder transmitted by autosomal recessive inheritance. It is caused by a mutation in the ABCB11 gene and characterized by intermittent episodes of severe cholestasis (minimum of two episodes of jaundice separated by symptom-free intervals of weeks up to years). The clinical presentation is less aggressive than PFIC. While PFIC starts during infancy or early childhood and often leads to liver cirrhosis,1 BRIC usually manifests later in life and has a more benign as well as recurrent pattern.3
PFIC are considered rare diseases and the actual prevalence is not known while the incidence is estimated to be one per 50.000-100.000 births.2 The diagnosis is basically based on the clinical history, age of onset, laboratory data, bile analysis, liver histology, anti-BSEP and anti-multidrug resistance P-glycoprotein (MDR) 3 immunostaining (respectively in PFIC 2 and PFIC3) and, in addition, with genetic analysis.
Histopathologically canalicular cholestasis, giant cell transformation, pronounced lobular/portal fibrosis and inflammation, are present.1 Treatment is mainly supportive with the use of high-dose ursodeoxycholic acid (UDCA) (25 mg per kilogram of body weight/daily)4 and fat-soluble vitamins (A, K, D, E) supplementation while rifampicin is used for itching.2 However some patients with PFIC2 may also benefit from biliary diversion5 and temporary endoscopic nasobiliary drainage (END) that should be maintained for a few weeks, to help selecting potential responders to biliary surgery.6,7 If these medical and surgical approaches fail, liver transplantation remains the only effective alternative, also from living-related donors.8,9 Over the last couple of years, in vitro and in vivo reports emerged about the effective use of the 4 phenyl-butyrate (4PB), that is normally used to treat ornithine trans-carbamylase deficiency, because has been demonstrated also to increase the hepatocanalicular expression of BSEP and the biliary excretion capacity of bile salts.10,11
Many drugs can cause cholestasis through interaction with hepatic transporters. To date, a relatively strong association between drug-induced cholestasis and attenuated BSEP activity has been proposed12 but BSEP deficiency are likely to be confused with drug induced liver injury (DILI).
Until now, more than 400 mutations in the three causing genes have been identified and a clinical continuum from mild to progressive phenotypes has been described.13-15 The molecular genetic diagnosis of PFIC is not currently widespread due the overlapping phenotypes and genetic heterogeneity of the disease as well as the laborious and expensive gene-sequencing methodology used so far. On the other hand Next Generation Sequencing (NGS) is a modern analysis system based on the massive parallel sequencing of specific genomic loci, whole exome or genomes. With respect to classic Sanger sequencing NGS allows rapid sequencing with more information at lower costs.16 For these reasons, some NGS-based protocols are recently proposed for the molecular diagnosis of PFIC.17,18
In literature only a few cases with intermediate phenotype between BRIC and PFIC or with progression from BRIC to PFIC are described, thus appearing low gGT cholestasis a clinical continuum.19-21
Herein we describe two cases, with late onset PFIC2, secondary to novel mutations in ABCB11 gene, identified by targeted-NGS.
Material and MethodsThe clinical diagnosis was suspected after at least two attacks of cholestasis, a normal anatomy of the biliary tract demonstrated with radiological investigations. All laboratory tests were negative, e.g., a normal serum gGT activity, negative viral markers, autoimmune and storage diseases were excluded and alfa1antitrypsin was found to be within range. Serum bile salts were assayed. The patients underwent following exams: abdominal ultrasounds, magnetic resonance cholangio-pancreatography (MRCP) et/or computed tomography (CT) abdomen, endoscopy, Fibro-Scan. Liver biopsy and immunostaining was performed with anti-BSEP antibody (goatpolyclonal antibody, 1:100 working dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After informed consent a genetic test was performed on both families.
NGS was performed using the Ion Torrent PGM System, according to manufacturer’s procedure (Life Technologies, CA, USA). A multiplex targeted-NGS approach (82 amplicons covering 98.23% of the 23,67 kb target sequences) was used for libraries preparation, allowing the simultaneous molecular analysis of ATP8B1, ABCB11 and ABCB4 genes. All mutations identified by NGS were confirmed by conventional sequencing.
The missense variant was evaluated for sequence conservation and the likelihood of pathogenicity using the bioinformatics tools PoliPhen2, Sorting Intolerant From Tolerant (SIFT) and Mutation Taster2.22-24
Case ReportCase 1 was a 19-year-old male, with a history of neonatal jaundice and hepatomegaly. In January 2013, at the age of 17 years, the patient was referred to our center for orthotopic liver transplantation evaluation. The mother was hepatitis C virus-infected and has a paternal uncle with a history of Gilbert syndrome. No family history of gallstones, intrahepatic cholestasis of pregnancy or early death in infancy was present. Growth disorders were not reported in childhood and dysmorphic features were absent. At the age of 13 years, jaundice recurred after an episode of fever and enteritis; total bilirubin was 156.4 μmol/L (normal range: < 19 μmol/L) and indirect bilirubin 153 μmol/L (< 5 μmol/L). The episode was mainly characterized by asthenia, pruritus and intestinal disorders (steatorrhea and hypocholic stools) but no definitive diagnosis was made. After two years a new episode of jaundice, with severe itching, recurred following upper respiratory tract infection. The bilirubin reached 345.4 μmol/L (direct 232.9 μmol/L), alanine (ALT) and aspartate aminotransferase (AST) was mildly elevated [(67 U/L (normal: < 40 U/L) and 56 (<40 U/l)], respectively while gGT and AP were within the normal range. A liver biopsy showed an intrahepatic cholestasis within a normal liver structure without fibrosis but immunostaining was not performed. Therefore, a form of progressive familial cholestasis was hypothesized and the patient was treated with UDCA and rifampicin. Liver biopsy was repeated after one year, the intrahepatic cholestasis persisted but a moderate fibrosis appeared.
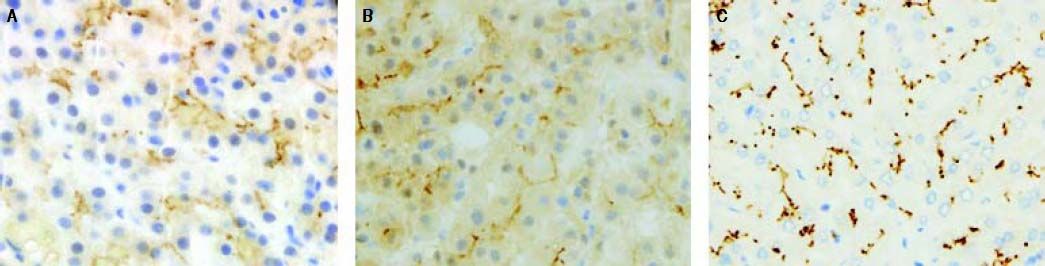
Anti-BSEP immune-staining produced an inhomogeneous pattern (reduced and focal) at the canalicular pole of hepatocytes while the anti-MDR3 immune-staining control was strong and diffuse (Figures 1A and 1C).
Anti-BSEP pattern at immunochemistry. A. In case 1 Anti-BSEP produced an inhomogeneous pattern of staining (reduced and focal) at the canalicular pole of hepatocytes. B. In case 2 a multifocal pattern of staining at the canalicular pole of hepatocytes was present. C. An anti-MDR3 immune-staining control was reported.
On physical examination, hepato-splenomegaly was noted while ascites and signs of encephalopathy were absent at the moment of the liver transplantation evaluation. Laboratory investigations revealed cholestasis with normal serum gGT. Hyperbilirubinemia was present (total 167.6 μmol/L/direct 155.6 μmol/L); ALT were 101 U/L, AST 163 U/L, International Normalized Ratio (INR) 1.27, albumin 5.8 μmol/L. Total bile acids ranged from 173 to 519 micromol/L (normal: < 10 μmol/L). Biliary tree was not dilated both on ultrasound scan and MRCP while abdominal CT scan excluded hepatocellular carcinoma and portal thrombosis. FibroScan was suggestive of severe fibrosis (47.2 kPa). Itching was controlled by UDCA (25 mg/kg body weight/daily) and rifampicin (6 mg/kg body weight/ daily).
During the follow up the patient’s general conditions improved. Bilirubin decreased slowly to reach 15 μmol/L. Transaminases normalized while the alkaline phosphatase persisted elevated. The patient was listed for liver transplantation with United Network for Organ Sharing (UNOS) 7 status and then temporally disabled.
After two years, in August 2014, jaundice reappeared and bilirubin increased to 247.8 μmol/L (direct 209.3 μmol/L). The stools were acholic and asthenia was present.
An experimental treatment with 4 PB, a clinically approved pharmacological chaperone, was started.10,11 The starting dose was 200 mg/kg body weight/daily in four assumptions but the drug was interrupted after two weeks for the appearance of severe side effects (irritability, mood swings, headache) without improvement of laboratory during treatment (still a suboptimal dose was used).
BSEP deficiency can benefit from END as described in some reports.6,7 So it was placed in November 2014 and maintained for 4 weeks. Bilirubin decreased to 109.4 μmol/L. Six months after END removal, bilirubin continued to decline (60 μmol/L), itching was absent and UNOS 7 status was maintained.
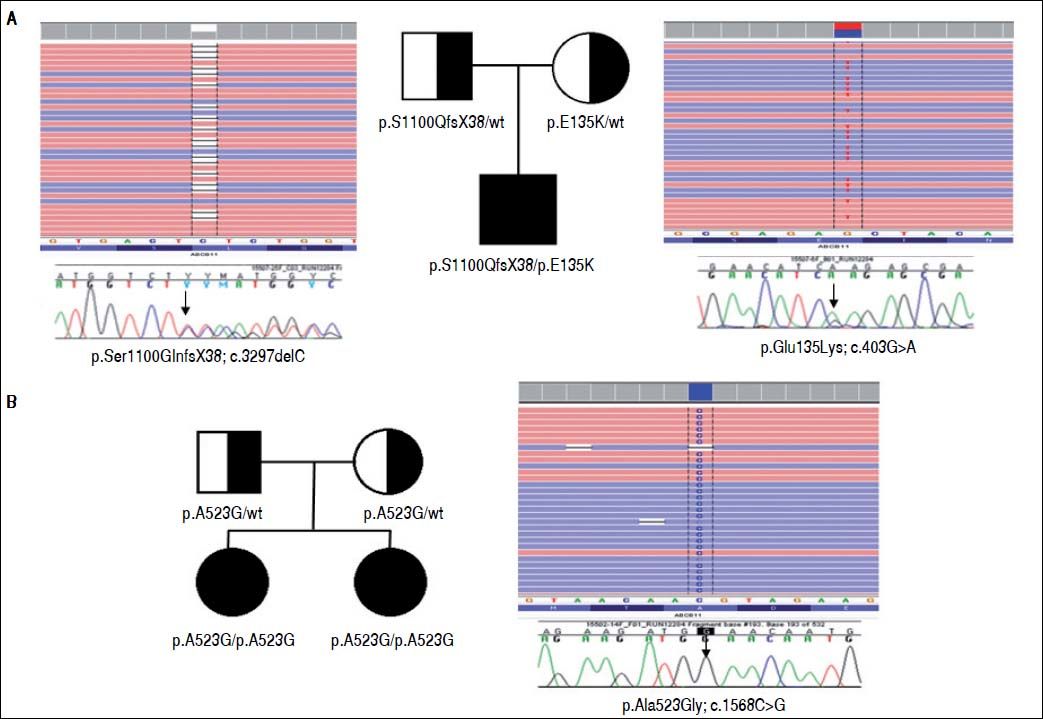
Two heterozygous defects in ABCB11 gene have been identified by NGS: the novel frameshift mutation p.Ser1100GlnfsX38 (c.3297delC) on exon 25, inherited through the paternal lineage, and the maternally inherited missense variant p.Glu135Lys (c.403G > A) on exon 6 (Figure 2A). The frameshift mutation predicts the formation of a truncated protein of 1138 aa (instead of 1321) and it is presumably pathogenic. The missense mutation has been described in pregnancy cholestasis and in BRIC, and it has been reported causing a reduction of mature protein.25,26
Family pedigrees, NGS reads and electropherograms. A. Mutations identified in Family of case 1. B. Mutations identified in Family of case 2. The family pedigrees are shown. The proband’s NGS reads (in reverse strand) and mutations are blasted on the human reference sequence and are shown by the Integrative Genomics Viewer (http://www.broadinstitute.org/igv/) on top. Sanger sequencing validation are shown below.
Case 2 was a 23-year-old female who was born at full term to unrelated parents. In June 2012, at the age of 20 years, the patient was referred to our centre for jaundice. At two months of age, she had an attack of jaundice, spontaneously resolved that lasted for 20 days. The patient had a positive family history for liver disorders. The younger sister, at the age of two years, experienced an attack of jaundice and itching requiring the hospitalization in a paediatric unit, a liver biopsy was performed demonstrating an intrahepatic cholestasis of unknown origin with a normal liver structure. The jaundice resolved spontaneously after a few weeks and the sister was discharged without indication of a regular monitoring. The patient had a normal childhood with no evidence of growth retardation. At twenty years of age, she started taking oral contraceptive pills (ethinyl-estradiol 0.020 mg plus drospirenone 3 mg). AST and ALT before starting therapy were normal. One month after the patient reported intense itching, jaundice, diarrhea with pale stools and dark urine, so contraceptive pills were discontinued. Liver tests showed hyperbilirubinemia (total 495.9 μmol/L, direct 461.7 μmol/L), AP 327 U/L (normal range: < 280 U/L) with a normal serum gGT activity (31 U/L), ALT 258 IU/L, AST 435 IU/L, INR 1.12, albumin 5.2 μmol/L, bile acids 100 μmol/L. Abdominal ultrasound and MRCP showed no biliary duct dilatation. FibroScan was suggestive of severe fibrosis (20.9 kPa). A liver biopsy showed severe intrahepatic cholestasis, portal tracts fibrosis with initial porto-portal bridging. Anti-BSEP showed a multifocal pattern of staining at the canalicular pole of hepatocytes, highly suggestive for BSEP deficiency (Figure 1B).
Therapy with daily cholestyramine (4 g/daily) and UDCA (15 mg/kg body weight/daily) were started with a slow reduction ofjaundice that completely resolved after 6 months. Using NGS approach (Figure 2B) the novel missense variant p.Ala523Gly (c.1568C>G) in exon 13 of ABCB11 gene was detected in the proband in homozygous form. This molecular defect was inherited from the parents, both heterozygous for the variant, and it was detected also in homozygous form in the sister. The segregation in the affected sister, as well as the bioinformatics predictions (SIFT: damaging; PolyPhen2: possibly damaging, Mutation Taster2: disease causing) indicated a causative role of this novel missense mutation.
After a mean follow up of three years, the patient is in good health without new episodes of jaundice unlike her sister who had an attack of hyperbilirubinemia at 9 years of age, in the course of therapy with amoxicillin for an upper respiratory tract infection.
During the follow up, laboratory investigations were within normal limits for bilirubin and transaminases while AP ranged from 415 to 325 U/L. The abdominal ultrasounds showed normal liver architecture.
DiscussionHerein we describe two patients who presented with atypical BSEP deficiency disease, due to novel mutations in ABCB11 gene. The molecular diagnosis was performed by parallel NGS of the PFIC genes ATP8B1, ABCB11 and ABCB4.
Traditionally BSEP deficiency is responsible for two phenotypes with different clinical course: PFIC2 represents an aggressive disease with permanent cholestasis that requires liver transplantation within the first years while BRIC2 is characterized by intermittent attacks of cholestasis but there is no progression to liver cirrhosis and life expectancy is good.
In literature, there are few cases of intermediate phenotype between BRIC and PFIC or forms progressing from BRIC to PFIC.19-21
Genetic mutations in ABCB11 gene, identified in the two families, are responsible for a similar clinical presentation. In the first case the disease is related to a compound heterozygosis of a frameshift and a missense mutation. The p.Glu135Lys mutation, already described in ICP and in BRIC, has been previously reported to cause a reduction of mature protein, while the novel p.Ser1100GlnfsX38 frameshift mutation, codifying an abnormal and shorter BSEP, could produce a non-functional protein. The co-presence of the aforementioned mutations can cause a BSEP deficiency disease of intermediate severity with a transitional phenotype between BRIC2 and PFIC2, with late onset and less aggressive course compared to the traditional PFIC.
Regarding the second patient the novel homozygous mutation of p.Ala523Gly can cause a similar form of late-onset disease triggered by certain drugs. Notably, the cholestatic attacks noted in the second case and her younger sister was triggered by medications (oral contraceptive pills and amoxicillin). Such forms ofjaundice can be easily misdiagnosed as DILI.
Liver biopsy is inconclusive for diagnosis. Immunochemistry in PFIC2 can be useful to confirm the suspect but only genetic testing poses definitive diagnosis. The current molecular analysis, however, is demanding, expensive and available only in few tertiary centres. Thus, intrahepatic familial cholestasis can be considered an underdiagnosed medical condition, mainly in chronic cholestasis of unknown aetiology or in atypical forms like in our two cases.
Therefore, our molecular analysis by NGS resulted an inexpensive and reliable methodology that can be implemented in disease-targeting sequencing and can help in diagnosing a disease whose course and prognosis are still far from defined. Furthermore, NGS permits rapid and simultaneous analysis of a large panel of cholestasis-associated genes and peculiar phenotypes that appear more likely to cause certain de novo pathogenic mutations. The clinical presentation and course of PFIC2 can be described as a continuum form mild to extreme late onset and severe disease.
In conclusion, we report two novel ABCB11 mutations responsible for an intermediate phenotype of BSEP deficiency disease that seem to be crossing between BRIC and PFIC. Moreover, we highlight the clinical utility of NGS in patients with cholestasis of unknown origin. Finally, intrahepatic cholestasis attacks in PFIC patients can be triggered by medications whereby drugs interfering with the BSEP function should be avoided.
Abbreviations- •
4PB: 4 phenyl-butyrate.
- •
ALT: alanine aminotransferase.
- •
AP: alkaline phosphatase.
- •
AST: aspartate aminotransferase.
- •
ATP: adenosine triphosphate.
- •
BRIC: benign intrahepatic cholestasis.
- •
BSEP: bile salt export pump.
- •
CT: computed tomography.
- •
DILI: drug induced liver injury.
- •
END: endoscopic nasobiliary drainage.
- •
gGT: gamma-glutamil-transpeptidase.
- •
INR: International Normalized Ratio.
- •
MDR: multidrug resistance P-glycoprotein.
- •
MRCP: magnetic resonance cholangio-pancreatography.
- •
NGS: next generation sequencing.
- •
PFIC: progressive familial intrahepatic cholestasis.
- •
UDCA: ursodeoxycholic acid.
- •
UNOS: United Network for Organ Sharing.
No grants and other financial support.
Author’s Contributions- •
Study concept: GV, PA, VM.
- •
Data collection: GV, VM, EM, AA, PF.
- •
Preparation of manuscript: GV, MP, EM.
- •
Critical review of manuscript: NG, FC, VM, PA.