Introduction and aim. Sarcopenia is an independent predictor of mortality in cirrhosis. Hypogonadism is common in cirrhosis and has been associated with sarcopenia in non-cirrhotic chronic liver disease populations. The aim of this study is to investigate if sarcopenia is associated with low testosterone levels in patients with cirrhosis.
Material and methods. This is a retrospective analysis of prospectively collected data of 211 cirrhotic patients undergoing evaluation for liver transplantation. Sarcopenia was defined by computed tomography (CT) scan using specific cutoffs of the 3rd lumbar vertebra skeletal muscle index (L3 SMI). Morning testosterone levels were obtained in all patients.
Results. Of the 211 patients, sarcopenia was noted in 94 (45%). Testosterone levels were lower in sarcopenic patients (10.7 ± 1.1 vs. 13.7 ± 1.4 nmol/L, p = 0.03) and hypotestosteronemia was more frequent in them too (34 vs. 16%, p = 0.004). In males, those with sarcopenia had lower testosterone levels (14.6 ± 1.4 vs. 21.9 ± 1.8, p = 0.002), and the corresponding frequency of hypotestosteronemia (42 vs. 19%, p = 0.006) was also higher. There were no significant differences in female patients. There was a weak correlation between L3 SMI and testosterone levels (r 0.37, p < 0.001). On multivariable regression analysis including sex, body mass index (BMI), hypotestosteronemia, MELD and etiology of cirrhosis, only hypotestosteronemia (RR 2.76, p = 0.005) and BMI (RR 0.88, p < 0.001) were independently associated with sarcopenia.
Conclusion. Low testosterone levels are associated with sarcopenia in male cirrhotic patients. The potential therapeutic effect of testosterone to reverse sarcopenia in these patients warrants evaluation in future trials.
Sarcopenia is one well-known complication of cirrhosis, it can be present in up to 45% of patients, and its prevalence increases as liver disease progresses.1 Myosteatosis, on the other hand, implies an increase in fatty infiltration within the skeletal muscle and is related to muscle quality. Both sarcopenia and myosteatosis, are independent predictors of mortality in this population.2,3
Some of the most important contributors to muscle wasting in cirrhosis are metabolic abnormalities, insufficient intake, malabsorption, impaired liver function to metabolize and endocrine abnormalities.4 Of the latter factors, low testosterone levels have been associated with sarcopenia in several chronic disease populations, including renal failure, heart failure, and obstructive pulmonary disease. The pharmacologic supplementation of this hormone has been shown to have anabolic effects on muscle mass in different populations.5,6 The relation between testosterone and myosteatosis has not been studied as thoroughly as that with sarcopenia, but in patients with prostate cancer, androgen deprivation therapy results in muscle fatty infiltration.7
Male patients with cirrhosis have lower testosterone levels than controls due to a central hypothalamus-pituitary mechanism, an increased peripheral aromatization of androgens and gonadal failure.8,9 In female patients with cirrhosis, fat depletion is likely to occur before a significant loss of muscle mass.4 Low testosterone levels are an independent predictor of mortality in cirrhosis.10,11 To the best of our knowledge, there have been no studies in patients with cirrhosis which have addressed the association between testosterone levels, sarcopenia, and myosteatosis, as determined by cross-sectional imaging, the current gold standard for estimating muscle mass.12
A clear association between these parameters would add to the evidence to consider testosterone as a therapeutic option for muscle wasting in cirrhosis. Accordingly, we aimed to investigate if sarcopenia and/or myosteatosis were associated with low testosterone levels in patients with cirrhosis.
Material and MethodsStudy populationTwo hundred and eleven patients were included in our retrospective analysis of prospectively collected data. Eligible subjects were cirrhotic patients who were seen in the liver units of the University of Alberta Hospital and the Foothills Medical Centre to assess their candidacy for liver transplantation between February 2011 and August 2012. The variables of interest were prospectively collected. Inclusion criteria were age between 18 to 80 years old and a diagnosis of cirrhosis confirmed by either histological (defined as presence of nodules of regeneration), radiological (ultrasound or cross-sectional imaging showing a lobulated liver and/or unequivocal signs of portal hypertension) or transient elastography (defined as liver stiffness ≥ 14 kPa) assessment. We excluded patients on any kind of hormonal replacement therapy, with extra-hepatic malignancy, end-stage renal disease on dialysis, chronic pulmonary disease requiring oxygen supplementation, or congestive heart failure with an ejection fraction < 40%. The study protocol was approved a priori by the institutional review board prior to data collection.
Muscularity, sarcopenia, and myosteatosis assessmentComputed tomography (CT) scans used for analysis were performed as part of the liver transplant evaluation. A transverse CT image from the third lumbar vertebra (L3) in the inferior direction was assessed from each scan. Images were analyzed with SliceOmatic V4.3 software (Tomovision, Montreal, Quebec, Canada), which enables specific tissue demarcation by using previously reported Hounsfield unit (HU) thresholds. Using this technique, skeletal muscle is identified and quantified by HU thresholds of -29 to +150. Muscles in the L3 region encompass psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis. The following HU thresholds were used for adipose tissues: -190 to -30 for subcutaneous and intermuscular adipose tissues, and -150 to -50 for visceral adipose tissues. Cross-sectional areas (cm2) were automatically computed by summing tissue pixels and multiplying by pixel surface area. All CT images were analyzed by 2 trained observers. Cross-sectional area of muscle and adipose tissue were normalized for stature (cm2/m2). The L3-SMI was expressed as cross-sectional muscle area/height. Sarcopenia was defined according to the following cutoffs: L3 Skeletal muscle index (L3 SMI): ≤ 41 cm2/m2 for women and ≤ 53 cm2/m2 for men with body mass index (BMI) ≥ 25 kg/m2 and ≤ 43 cm2/m2 in all patients with a BMI < 25 kg/m2.3 Muscle attenuation analysis was performed in 87 patients (41%). Myosteatosis was defined according to cut-off values for muscle attenuation that have been associated with mortality, specifically: < 41 HU in patients with a BMI up to 24.9, and < 33 in those with a BMI ≥ 25.13
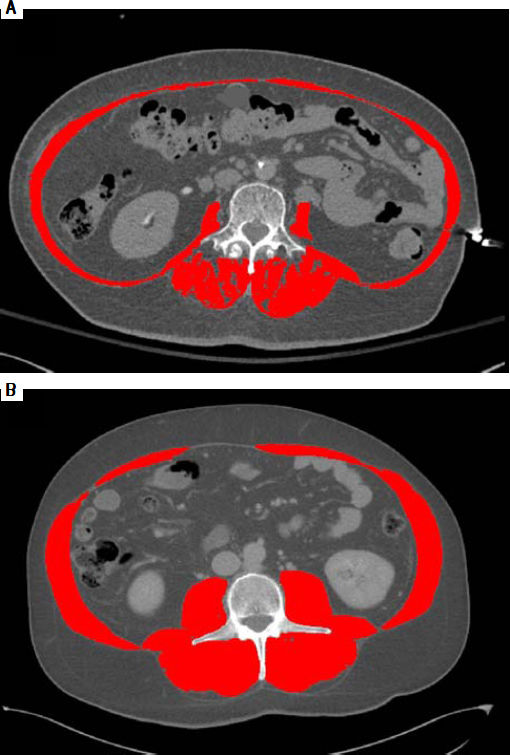
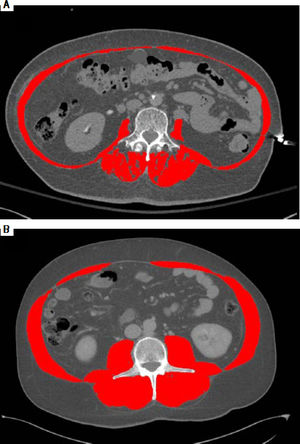
As an example of the L3 SMI assessment, figures 1A and 1B illustrate two cirrhotic patients with similar BMI (26 kg/m2). Abdominal CT images in horizontal plane are images taken at L3, the red color indicates skeletal muscles. CT at the left (1A) is from a male patient with sarcopenia with L3 SMI 38 cm2/m2, and the CT at the right (1B) is from a non-sarcopenic male with a L3 SMI of 56 cm2/m2.
Clinical and laboratory assessmentWe collected the following information: morning testosterone levels, age, gender, etiology of cirrhosis, and liver tests. Testosterone levels were reported in nmol/L (normal males: 10.3-29.5; females: 0.6-2.0) with values below the lower limit of normally, considered as hypotestosteronemia. Testosterone in all samples was measured with an electrochemiluminescence immunoassay (ECLIA). Standard commercial kits (Elecsys® Testosterone II, Roche Diagnostics, Mannheim, Germany) were used and the mean ± SD of the testosterone levels (ng mL-1) were reported. The sensitivity of the assay was 0.04 ng mL-1 for testosterone. The clinical and biochemical parameters were obtained within 1 week from the index CT scan used to determine the L3 SMI.
Statistical analysesContinuous data were described using means and standard deviations; for categorical data we used absolute and relative frequencies. For bivariate analysis, for continuous and categorical variables we used the χ2 test and the unpaired t test respectively. The Pearson’s correlation coefficient was used to evaluate the correlation between testosterone levels and L3 SMI. For multiple logistic regression analysis, we considered sarcopenia as the dependent variable. Biochemical parameters (sodium, creatinine, albumin, INR, testosterone levels), age, etiology of cirrhosis, and sex, were entered as independent variables. Variables of interest plus variables with a p-value < 0.1 in univariate analysis were included in multivariable regression analysis. We also used logistic regression to evaluate the association between myosteatosis, as a dependent variable, testosterone, and sarcopenia. Statistical analysis was performed using SPSS 22 (SPSS, Inc., Chicago, IL).
ResultsClinical and Biochemical Features of Patients EvaluatedOf the 211 patients, 128 patients were male (61%), and the mean age was 57 ± 1 years. Cirrhosis was caused by hepatitis C virus infection (HCV) in 74 (35%), alcohol in 54 (26%), non-alcoholic fatty liver disease (NAFLD) / cryptogenic in 53 (25%), autoimmune liver disease in 18 (9%), and other etiology in 11 patients (5%).
The mean testosterone level in males was 18.5 ± 1.2 nmol/L, and in females 1.5 ± 0.1 nmol/L. Hypotestosteronemia was present in 51 (24%) patients. Thirty-eight male patients (30%), and 13 female patients (16%) had levels below the reference threshold for our laboratory (p = 0.03).
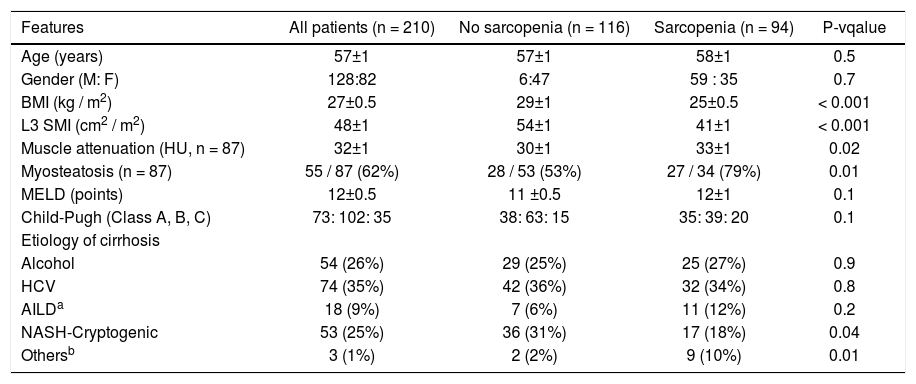
Clinical and biochemical features overall and according to the presence of sarcopenia are shown in table 1.
Features associated with sarcopenia in patients with cirrhosis.
| Features | All patients (n = 210) | No sarcopenia (n = 116) | Sarcopenia (n = 94) | P-vqalue |
|---|---|---|---|---|
| Age (years) | 57±1 | 57±1 | 58±1 | 0.5 |
| Gender (M: F) | 128:82 | 6:47 | 59 : 35 | 0.7 |
| BMI (kg / m2) | 27±0.5 | 29±1 | 25±0.5 | < 0.001 |
| L3 SMI (cm2 / m2) | 48±1 | 54±1 | 41±1 | < 0.001 |
| Muscle attenuation (HU, n = 87) | 32±1 | 30±1 | 33±1 | 0.02 |
| Myosteatosis (n = 87) | 55 / 87 (62%) | 28 / 53 (53%) | 27 / 34 (79%) | 0.01 |
| MELD (points) | 12±0.5 | 11 ±0.5 | 12±1 | 0.1 |
| Child-Pugh (Class A, B, C) | 73: 102: 35 | 38: 63: 15 | 35: 39: 20 | 0.1 |
| Etiology of cirrhosis | ||||
| Alcohol | 54 (26%) | 29 (25%) | 25 (27%) | 0.9 |
| HCV | 74 (35%) | 42 (36%) | 32 (34%) | 0.8 |
| AILDa | 18 (9%) | 7 (6%) | 11 (12%) | 0.2 |
| NASH-Cryptogenic | 53 (25%) | 36 (31%) | 17 (18%) | 0.04 |
| Othersb | 3 (1%) | 2 (2%) | 9 (10%) | 0.01 |
AILD: autoimmune liver disease. BMI: body mass index. L3 SMI: lumbar 3rd skeletal muscle index. HCV: hepatitis C virus. MELD: model for end stage liver disease. NASH: non-alcoholic steatohepatitis.
Mean L3 SMI was 48 ± 1 cm2/m2 and was higher in male than in female patients (52 ± 1 vs. 43 ± 1 cm2/m2, p < 0.001). Sarcopenia was noted in 94 patients (45%): 59 male (46%) and 35 female (43%) patients (p = 0.7). Patients with sarcopenia had lower BMI (25 ± 0.5 vs. 29.1 ± 1 kg/m2, p < 0.001). In terms of the etiology of cirrhosis, the prevalence of NAFLD/cryptogenic was lower in patients with sarcopenia (18 vs. 31%, p = 0.04).
Hypotestosteronemia was more prevalent in sarcopenic patients (34 vs. 16%, p = 0.004) (Table 1).
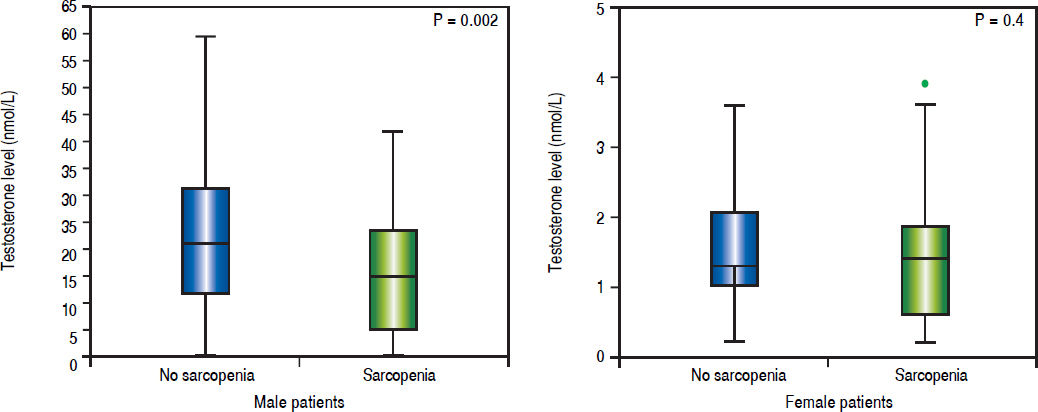
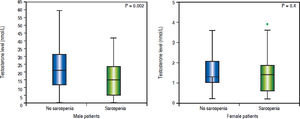
Sexual dimorphism in sarcopenia and hypotestosteronemiaMale sarcopenic patients had a lower BMI (26 ± 1 vs. 28 ± 1 kg/m2, p = 0.04) and lower testosterone levels when compared to non-sarcopenic patients (14.6 ± 1.4 vs. 21.9 ± 1.8 nmol/L, p = 0.002) (Table 2). Also, the prevalence of hypotestosteronemia (42 vs. 19%, p = 0.006) was higher in sarcopenic than non-sarcopenic patients (Table 2; Figure 2A).
Features associated with sarcopenia stratified by gender - univariate regression analysis.
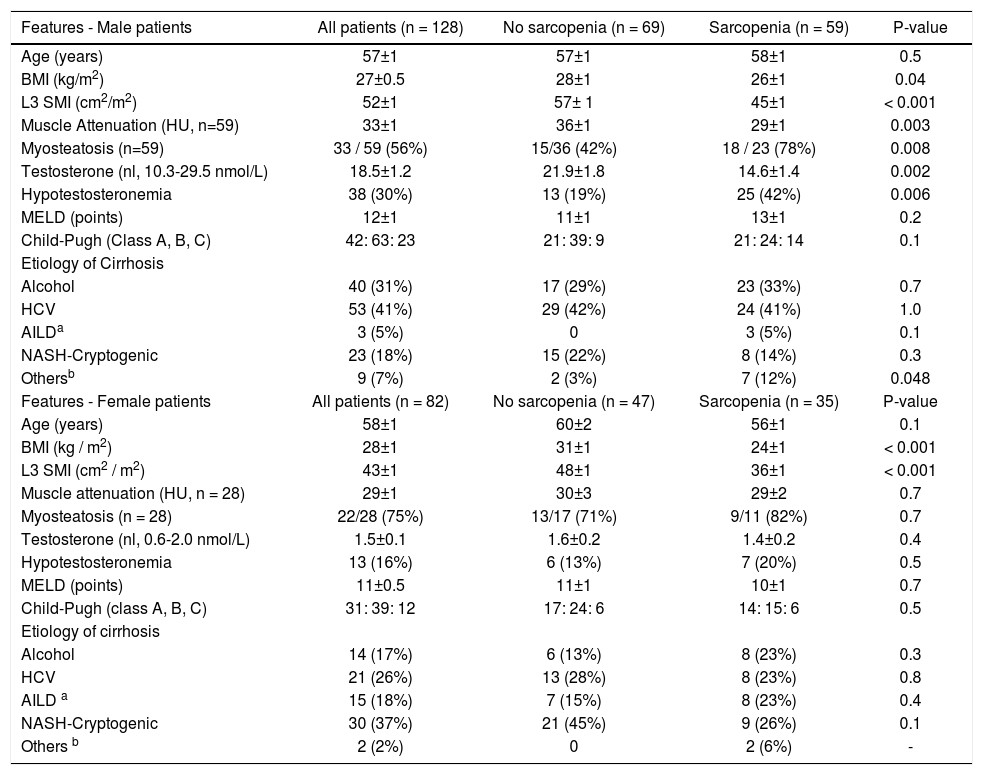
| Features - Male patients | All patients (n = 128) | No sarcopenia (n = 69) | Sarcopenia (n = 59) | P-value |
|---|---|---|---|---|
| Age (years) | 57±1 | 57±1 | 58±1 | 0.5 |
| BMI (kg/m2) | 27±0.5 | 28±1 | 26±1 | 0.04 |
| L3 SMI (cm2/m2) | 52±1 | 57± 1 | 45±1 | < 0.001 |
| Muscle Attenuation (HU, n=59) | 33±1 | 36±1 | 29±1 | 0.003 |
| Myosteatosis (n=59) | 33 / 59 (56%) | 15/36 (42%) | 18 / 23 (78%) | 0.008 |
| Testosterone (nl, 10.3-29.5 nmol/L) | 18.5±1.2 | 21.9±1.8 | 14.6±1.4 | 0.002 |
| Hypotestosteronemia | 38 (30%) | 13 (19%) | 25 (42%) | 0.006 |
| MELD (points) | 12±1 | 11±1 | 13±1 | 0.2 |
| Child-Pugh (Class A, B, C) | 42: 63: 23 | 21: 39: 9 | 21: 24: 14 | 0.1 |
| Etiology of Cirrhosis | ||||
| Alcohol | 40 (31%) | 17 (29%) | 23 (33%) | 0.7 |
| HCV | 53 (41%) | 29 (42%) | 24 (41%) | 1.0 |
| AILDa | 3 (5%) | 0 | 3 (5%) | 0.1 |
| NASH-Cryptogenic | 23 (18%) | 15 (22%) | 8 (14%) | 0.3 |
| Othersb | 9 (7%) | 2 (3%) | 7 (12%) | 0.048 |
| Features - Female patients | All patients (n = 82) | No sarcopenia (n = 47) | Sarcopenia (n = 35) | P-value |
| Age (years) | 58±1 | 60±2 | 56±1 | 0.1 |
| BMI (kg / m2) | 28±1 | 31±1 | 24±1 | < 0.001 |
| L3 SMI (cm2 / m2) | 43±1 | 48±1 | 36±1 | < 0.001 |
| Muscle attenuation (HU, n = 28) | 29±1 | 30±3 | 29±2 | 0.7 |
| Myosteatosis (n = 28) | 22/28 (75%) | 13/17 (71%) | 9/11 (82%) | 0.7 |
| Testosterone (nl, 0.6-2.0 nmol/L) | 1.5±0.1 | 1.6±0.2 | 1.4±0.2 | 0.4 |
| Hypotestosteronemia | 13 (16%) | 6 (13%) | 7 (20%) | 0.5 |
| MELD (points) | 11±0.5 | 11±1 | 10±1 | 0.7 |
| Child-Pugh (class A, B, C) | 31: 39: 12 | 17: 24: 6 | 14: 15: 6 | 0.5 |
| Etiology of cirrhosis | ||||
| Alcohol | 14 (17%) | 6 (13%) | 8 (23%) | 0.3 |
| HCV | 21 (26%) | 13 (28%) | 8 (23%) | 0.8 |
| AILD a | 15 (18%) | 7 (15%) | 8 (23%) | 0.4 |
| NASH-Cryptogenic | 30 (37%) | 21 (45%) | 9 (26%) | 0.1 |
| Others b | 2 (2%) | 0 | 2 (6%) | - |
AILD: autoimmune liver disease. BMI: body mass index. L3 SMI: lumbar 3rd skeletal muscle index. HCV: hepatitis C virus. MELD: model for end stage liver disease. NASH: non-alcoholic steatohepatitis.
There were no significant differences in testosterone levels between female sarcopenic and non-sarcopenic patients (1.6 ± 0.2 vs. 1.4 ± 0.2 nmol/L, p = 0.4). Also, there was no significant difference in the frequency of hypotestosteronemia among sarcopenic and non-sarcopenic female patients (20 vs. 13%, p = 0.5) (Table 2; Figure 2B).
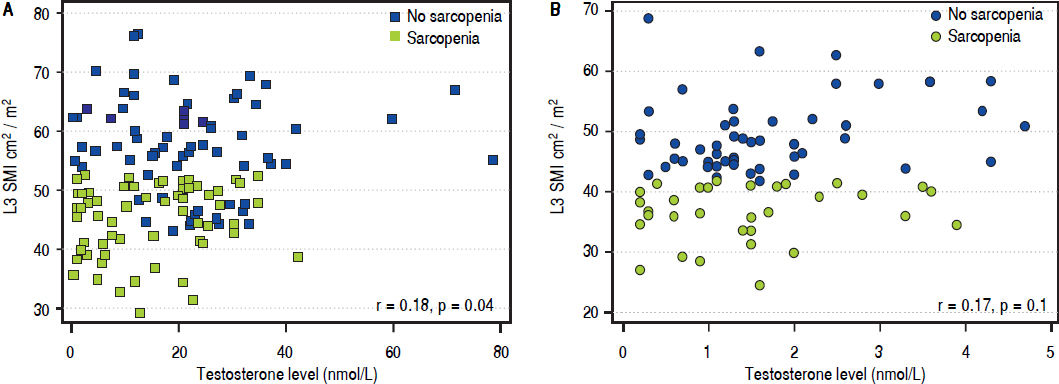
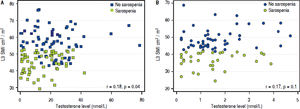
Correlations and factors associated with sarcopenia by multivariable regression analysisThere was a significant but moderate correlation between the L3 SMI and testosterone levels (r = 0.37, p < 0.001. The correlation was still significant, but weaker, in male patients (r = 0.18, p = 0.04), and was not significant in female patients (r = 0.17, p = 0.1) (Figure 3).
By multivariable regression analysis including sex, BMI, hypotestosteronemia, MELD score, and etiology of the cirrhosis, only hypotestosteronemia (RR = 2.76, p = 0.005) and BMI (RR = 0.88, P < 0.001 were independently associated with sarcopenia. These results were replicated in a model that included only male patients, whereas in female patients only BMI was associated with sarcopenia (Table 3).
Features associated with sarcopenia by multivariable regression analysis.
| Features | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Gender | 1.14 | 0.65 - 2.01 | 0.6 | 0.85 | 0.45 - 1.61 | 0.6 |
| BMI (kg/m2) | 0.86 | 0.81 - 0.92 | < 0.001 | 0.86 | 0.81 - 0.92 | < 0.001 |
| Hypotestosteronemia | 2.63 | 1.37 - 5.05 | 0.004 | 2.49 | 1.23 - 5.01 | 0.01 |
| MELD | 1.04 | 0.98 - 1.09 | 0.12 | - | - | - |
| NASH-Cryptogenic cirrhosis | 0.49 | 0.25 - 0.94 | 0.03 | 0.66 | 0.32-1.38 | 0.2 |
| Male patients | ||||||
| BMI (kg/m2) | 0.92 | 0.86 - 0.99 | 0.04 | 0.93 | 0.85 - 1.00 | 0.04 |
| Testosterone (nmol/L) | 0.95 | 0.92 - 0.98 | 0.004 | 0.95 | 0.98 - 0.98 | 0.004 |
| MELD | 1.04 | 0.98 - 1.10 | 0.16 | - | - | - |
| NASH - Cryptogenic cirrhosis | 0.56 | 0.22 - 1.44 | 0.2 | - | - | - |
| Female patients | ||||||
| BMI (kg / m2) | 0.77 | 0.98 - 0.86 | < 0.001 | 0.77 | 0.68 - 0.87 | < 0.001 |
| Testosterone (nmol / L) | 0.83 | 0.54 - 1.27 | 0.3 | - | - | - |
| MELD | 1.02 | 0.92 - 1.12 | 0.6 | - | - | - |
| NASH - Cryptogenic cirrhosis | 0.43 | 0.16 - 1.10 | 0.08 | 0.57 | 0.18 - 1.82 | 0.3 |
BMI: body mass index. CI: confidence interval. RR: relative risk. MELD: model for end stage liver disease. NASH: non-alcoholic steatohepatitis.
The attenuation index for determination of myosteatosis was available in 87 patients; of these, 55 (63%) had myosteatosis. It was more commonly identified in sarcopenic than in non-sarcopenic patients (79% vs. 53%, p = 0.01). Although myosteatosis was more common in male patients with sarcopenia (78 vs. 42%, p = 0.008), this was not the case in female patients with sarcopenia (82% vs. 71%, p = 0.7). By multiple regression analysis, myosteatosis was associated with sarcopenia (RR 2.85, p = 0.046), but not with hypotestosteronemia (RR 2.18, p = 0.1).
DiscussionIn this study, we demonstrate an association between testosterone levels and sarcopenia. This association was independent of well-known factors that contribute to testosterone levels, such as age, the stage of liver disease, and the etiology of cirrhosis.
Sarcopenia in cirrhosis is an independent predictor of mortality before transplantation2 and morbidity after liver transplantation.14 Currently, cross-sectional imaging studies, including CT scan or MRI, are the gold standard tools to quantify skeletal muscle mass and hence constitute a good resource for objective, detailed and reproducible nutritional assessment of patients.12 A meta-analysis evaluating the impact of CT-assessed sarcopenia including 19 studies, with more than 3,000 patients, showed an independent association between sarcopenia and waiting list and post-liver transplant mortality.15 More recently, a multicenter study across five academic transplant centers in North America including almost 400 adult patients with cirrhosis listed for liver transplant validated the association of sarcopenia and mortality and established similar cutoff values in cirrhotic patients awaiting liver transplantation using L3 SMI.16
Hypotestosteronemia is one of several mechanisms implicated in sarcopenia in cirrhosis.1 We found testosterone levels were significantly lower in male patients with sarcopenia, but the correlation between L3-SMI and testosterone levels was weak, suggesting that there are many factors implicated in sarcopenia and that the degree of hypotestosteronemia is not intimately associated with the degree of muscle wasting. These findings are consistent with the results of recent studies by Sinclair et al. that demonstrated also a weak, but significant correlation between testosterone levels and muscle mass, and that low testosterone levels were associated with higher mortality in male patients with cirrhosis.11,17 We did not find this association in females. The absolute difference between testosterone levels in sarcopenic and non-sarcopenic females was small (0.2 vs. 7.3 nmol/L in males). Moreover, as only 39% of our sample was comprised of women and only 16% of them had hypotestosteronemia, it is possible that our study was underpowered to evaluate this association in female patients. Studies in postmenopausal women have shown that low testosterone levels are associated with a lower muscle mass.18 In addition to this, we used an immunoassay to determine testosterone levels, which is known to have reduced sensitivity in case of female patients, who usually have much lower concentrations when compared to males, further limiting the interpretation of the results found in women. The association between sarcopenia and hypotestosteronemia in women requires evaluation in future studies.
Sarcopenia in liver cirrhosis is multifactorial; hence, it is improbable that a single treatment will be able to reverse it.1 Treatment approaches so far have focused on nutritional supplementation, replacing calories and proteins by different routes of administration, with limited benefit, suggesting that cirrhosis is a state of anabolic resistance. Exercise and physical activity may be of benefit but are limited by fatigue in this population.19,20 The significant association between sarcopenia and testosterone and the data from recent supplementation trials in cirrhosis21,22 indicate that replacement therapy may be a potential therapeutic strategy to improve muscle mass. Additionally, testosterone has the advantage of improving bone mineral density, which could be of much benefit in patients with cirrhosis, who have an increased risk of fractures.23 Future testosterone replacement studies in cirrhosis will be needed to confirm the effect of supplementation not only on muscle mass but also on muscle quality, to assess whether there may also be a role for testosterone supplementation in female patients, and to study the safety of such intervention. There are several potential adverse effects that have been associated with testosterone supplementation. Observational studies have linked testosterone levels with hepatocellular carcinoma, but results have been inconsistent.24–26 There were no hepatocellular carcinoma cases in the clinical trials mentioned earlier, but these were small studies probably insufficiently powered to study that outcome.21,22 When using testosterone replacement therapy to increase bone mineral density, clinical guidelines on the management of osteoporosis in cirrhosis recommend discussing risks/benefits with the patient before starting therapy.27 Testosterone replacement is not recommended in patients with prostate cancer, erythrocytosis, hyperviscosity, sleep apnea, or heart failure.18,28,29 In addition to this, as suggested by the European Working Group on Sarcopenia in Older People (EWGSOP),30 it may be inappropriate to define sarcopenia only considering mass, as there is a nonlinear correlation between muscle mass and function, so therapies exclusively focused on muscle mass may turn to be ineffective unless some strategies are considered to improve function as well.1,4
Myosteatosis has been recognized as an independent predictor of long-term mortality in patients with liver cirrhosis.3 There is fewer data about the association between fatty infiltration of the muscle and testosterone. In patients with prostate cancer, androgen deprivation therapy has been associated with myosteatosis.7 Despite hypotestosteronemia being more frequent in patients with myosteatosis, the difference was not statistically significant. Of note, we could assess muscle quality in a limited number of patients, so the interpretation of this result must be done with caution, as a type 2 error cannot be ruled out. The association between testosterone and myosteatosis and the role of testosterone replacement in improving muscle quality in this population is largely unknown and will need to be assessed in future trials.
Our study has both strengths and limitations. To our knowledge, this is the first study that has evaluated the association between testosterone levels and sarcopenia in both male and female patients with cirrhosis, and also the first to assess the relation between myosteatosis and testosterone. Moreover, muscle mass was determined by cross-sectional imaging, which is currently considered by some as the gold standard in this population.12 Although we have shown that there is a significant association between testosterone levels and sarcopenia in male patients, due to the lack of a randomized interventional study design we are unable to draw conclusions about causality. We also did not determine other hormone levels which may play a role in this association such as estrone, estradiol, prolactin and, free testosterone. In fact, results from other studies have been inconsistent about whether free testosterone or total testosterone should be measured in these patients and some studies suggest that estrone and the testosterone/estradiol might also have clinical and prognostic implications. Patients with compensated cirrhosis have higher levels of the sex hormone binding globulin when compared with healthy controls, while patients that are decompensated have low levels, suggesting free testosterone may be a more reliable marker. However, the equations that are normally used to indirectly calculate free testosterone, have not been validated in patients with cirrhosis.21,24,31 In the case of women patients, because of their lower testosterone levels, the sensitivity of immunoassays is compromised and some authors consider it completely unreliable. However, the assay that we used has been shown to have good agreement with liquid chromatography – mass spectrometry.32 Another limitation is that we could only retrieve the information about myosteatosis in 41% of the patients, which may limit the interpretation of this result.
In conclusion, low testosterone levels are associated with sarcopenia in male patients with cirrhosis. Testosterone replacement therapy might be a potential therapeutic strategy to improve muscle mass in male cirrhotic patients, and its role in improving muscle quality remains to be defined. The safety and efficacy of this treatment require further prospective evaluation.
Abbreviations- •
BMI: body mass index.
- •
CT: computed tomography.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HU: hounsfield unit.
- •
L3: third lumbar vertebra.
- •
L3 SMI: L3 skeletal muscle index.
- •
NAFLD: non-alcoholic fatty liver disease.
Carlos Moctezuma-Velázquez, Gavin Low, Marina Mourtzakis, Mang Ma, Kelly W. Burak, Aldo J. MontanoLoza, and Puneeta Tandon declare no conflict of interest.
GrantsCarlos Moctezuma-Velazquez received a grant from Gilead Canada to complete his hepatology fellowship.
AcknowledgmentsPresented in part at the International Liver Congress of the European Association for the Study of Liver Diseases, April 24, 2015; Vienna, Austria.