Introduction and aim. 1. Study of liver explants - Etiologic types of end-stage chronic liver disease (ESCLD) and acute liver failure (ALF) in adults and children. 2. Assessment of donor steatosis and incidental granulomas. 3. Post-transplant liver biopsies.
Material and methods. Specimens of 180 explant hepatectomies, 173 donor wedge and 30 core liver biopsies, and 58 post transplant liver biopsies received in our department from April 2013 to March 2017.
Results. 1. Most common causes of ESCLD in adults were: alcohol related (30.32%), hepatitis virus related (18.71%) and non-alcoholic steatohepatitis related (18.06%); and in children < 12 years were: biliary atresia (27.27%), autoimmune disease (18.18%) and Wilson’s disease (18.18%). Most common causes of ALF in adults and children were anti-tubercular therapy induced and idiopathic, respectively. 2. Prevalence rate of moderate steatosis (between 30-60%) was 4.28%. Incidental granulomas were seen in 5 cases. 3. Most common diagnoses of post-transplant biopsies in adults included acute cellular rejection (ACR) (36.17%), recurrence of viral disease (8.51%) and moderate non-specific portal triaditis (8.51%). Among children < 12 years, most common diagnoses included unremarkable liver parenchyma, ACR and ischemia/reper fusion injury.
Conclusion. 1. Alcohol- and hepatitis- virus related ESCLD, and biliary atresia are leading indications for liver transplantation in adults and children, respectively. 2. Prevalence of 4.28% of moderate steatosis, is much lower than that documented in western literature. Only 5 cases of incidental granulomas is unexpectedly low in a country endemic for tuberculosis. 3. Most common diagnoses of post-transplant liver biopsies in adults has been acute rejection, which is similar to the findings from much larger published series.
Chronic liver disease (CLD) contributes significantly to the burden of disease worldwide. Failure to detect and treat these diseases early, may cause a number of them to progress to an irreversible stage, which may not be amenable to treatment and often lead to fatal complications. Special attention should be given to the evaluation of ESCLD in infants and children, as many of the etiologies are distinct from the conditions affecting the adult population. Liver transplantation (LT) has proven to be an effective modality to prolonging a better quality of life in many patients with end stage CLD.
While the history of orthotopic LT in India is relatively short, the program has steadily progressed, in no small part due to the efforts to increase awareness among the general population about liver disease and the scarcity of donors.
The aim of our study is threefold: 1) To attempt an etiological categorization of cases of end stage CLD and acute liver failure separately in adults and children ≤ 12 years, through an extensive pre-LT clinical, laboratory and radiological examination and thorough examination of the explant livers. 2) To assess the donor liver for prevalence of steatosis and unexpected incidental findings, like granulomas. 3) Analysis of post-transplant liver biopsies to establish the cause of graft dysfunction.
Material and MethodsDuring 4 year period from April 2013 to March 2017, 180 patients underwent LT in our institute.
Patients were selected based on the Child-Turcotte-Pugh classification, Model for End Stage Liver Disease score and Pediatric End-Stage liver disease score for CLD and the King’s College criteria for hepatic failure.1–4
Expiant liversPre-LT categorization of the etiologic type of CLD and acute liver failureWas done on the basis of clinical and investigational data. These included a history of alcohol consumption, drug intake, blood transfusion and intake of medication. Serology for hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infection, an autoantibody profile including antinuclear, anti-smooth muscle and anti-mitochondrial antibodies, and tests for serum levels of iron, ferritin and ceruloplasmin were carried out. Imaging studies were performed to detect neoplastic lesions.
In pediatric patients, a detailed family history was elicited and a thorough physical examination was performed, in addition to laboratory tests. Imaging modalities were helpful in cholestatic liver disease to ascertain the etiology.
Morphological evaluationWas carried out after serially sectioning the explant livers at 1 cm intervals, adequate fixation in 10% neutral buffered formalin and representative sampling. Four sections from the right lobe, 2 sections from the left lobe and 1 section from the hilum were studied in each case. Paraffin sections were stained by haematoxylin and eosin (H & E), and when required, by special stains for collagen, reticulin, iron and copper.
The microscopic evaluation included the type of nodules (macro vs. micro), fibrosis, inflammation and the type of inflammatory cells, steatosis, Mallory hyaline bodies, necrosis, bile ductular proliferation, deposition of iron or copper pigment and liver tumors.
In cases of acute liver failure, emphasis was laid upon the zone of necrosis and the integrity of the reticulin framework.
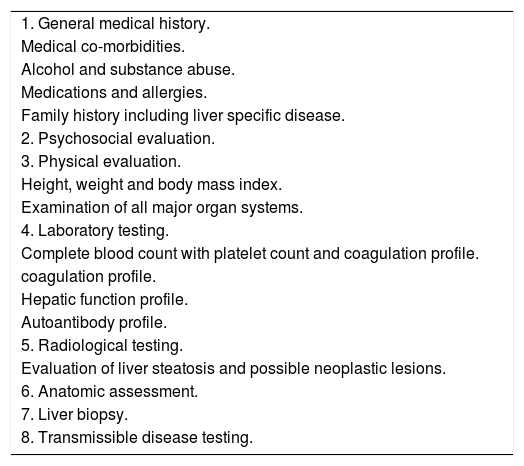
Donor biopsiesWhile no randomised controlled trials have been conducted to determine the tests required to evaluate a living donor, we follow the general medical practice of existing transplant programs5 (Table 1). All living donors fell into the age group of 18-60 years and were required to be related to the recipient, vide the Transplantation of Human Organs and Tissues Rules, 2013 published by the Ministry of Health and Family welfare, Government of India.
Evaluation of a living donor.
| 1. General medical history. |
| Medical co-morbidities. |
| Alcohol and substance abuse. |
| Medications and allergies. |
| Family history including liver specific disease. |
| 2. Psychosocial evaluation. |
| 3. Physical evaluation. |
| Height, weight and body mass index. |
| Examination of all major organ systems. |
| 4. Laboratory testing. |
| Complete blood count with platelet count and coagulation profile. |
| coagulation profile. |
| Hepatic function profile. |
| Autoantibody profile. |
| 5. Radiological testing. |
| Evaluation of liver steatosis and possible neoplastic lesions. |
| 6. Anatomic assessment. |
| 7. Liver biopsy. |
| 8. Transmissible disease testing. |
173 intra-operative wedge and 30 preoperative core liver biopsies from donors were analysed for the prevalence of steatosis. The indications for pre-LT core liver biopsies included steatosis on Plain CT scan, with a liver attenuation index (LAI) < 5, abnormal liver function tests or a body mass index (BMI) > 30.
The samples were adequately fixed, stained with H & E stain and the percentage of steatosis was calculated by two consultant pathologists (Dr. MH and Dr. BK).
Also incidentally detected were epithelioid granulomas in 5 wedge biopsies from asymptomatic donors.
Post-transplant liver biopsies58 post-transplant liver biopsies from both adult and pediatric recipients were studied after appropriate processing & staining with H & E. Special stains for collagen, reticulin, hemosiderin, etc. and immunohistochemistry for C4d and CK 7 were done when indicated.
The following features were evaluated in the liver biopsies:
- •
Adequacy of the biopsy, based on the number of portal tracts (biopsies with at least 5 portal tracts were considered adequate).
- •
Liver architecture.
- •
Presence of features of acute or chronic rejection, recurrence of original disease, biliary obstruction, infection, malignancy, etc.
Grading in cases of rejection was according to the Banff schema.6
ResultsExplant liversThe 180 patients in this study included 167 adults and 13 children ≤ 12 years of age. Among the adult patients, there were 134 males and 33 females, with the oldest recipient being 70 years old. Among the pediatric patients, there were 5 males and 8 females, ranging in age from 8 months to 12 years.
Of the 167 adult patients, 28 received whole livers from cadaveric donors, while the remaining 139 underwent living donor LTs. All the pediatric patients underwent living donor LTs.
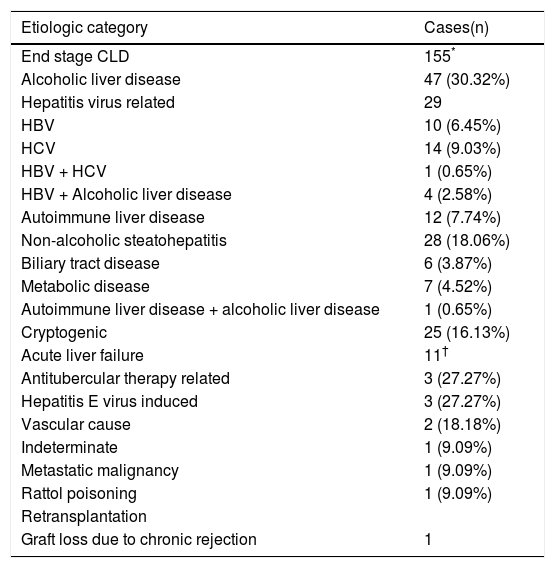
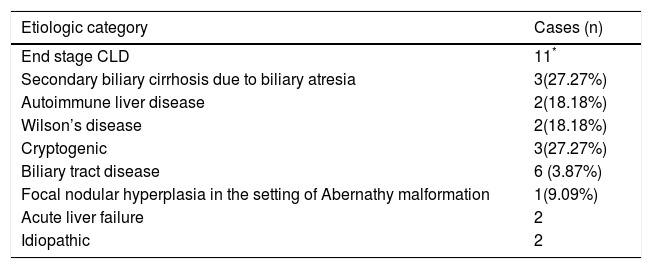
The etiologic categorisation of end stage CLD and acute liver failure in our study for the adult and pediatric cohorts has been summarised in tables 2 and 3.
Etiologic categorisation of end stage CLD and acute liver failure in adults.
| Etiologic category | Cases(n) |
|---|---|
| End stage CLD | 155* |
| Alcoholic liver disease | 47 (30.32%) |
| Hepatitis virus related | 29 |
| HBV | 10 (6.45%) |
| HCV | 14 (9.03%) |
| HBV + HCV | 1 (0.65%) |
| HBV + Alcoholic liver disease | 4 (2.58%) |
| Autoimmune liver disease | 12 (7.74%) |
| Non-alcoholic steatohepatitis | 28 (18.06%) |
| Biliary tract disease | 6 (3.87%) |
| Metabolic disease | 7 (4.52%) |
| Autoimmune liver disease + alcoholic liver disease | 1 (0.65%) |
| Cryptogenic | 25 (16.13%) |
| Acute liver failure | 11† |
| Antitubercular therapy related | 3 (27.27%) |
| Hepatitis E virus induced | 3 (27.27%) |
| Vascular cause | 2 (18.18%) |
| Indeterminate | 1 (9.09%) |
| Metastatic malignancy | 1 (9.09%) |
| Rattol poisoning | 1 (9.09%) |
| Retransplantation | |
| Graft loss due to chronic rejection | 1 |
Etiologic categorisation of end stage CLD and acute liver failure in children ≤ 12 years.
| Etiologic category | Cases (n) |
|---|---|
| End stage CLD | 11* |
| Secondary biliary cirrhosis due to biliary atresia | 3(27.27%) |
| Autoimmune liver disease | 2(18.18%) |
| Wilson’s disease | 2(18.18%) |
| Cryptogenic | 3(27.27%) |
| Biliary tract disease | 6 (3.87%) |
| Focal nodular hyperplasia in the setting of Abernathy malformation | 1(9.09%) |
| Acute liver failure | 2 |
| Idiopathic | 2 |
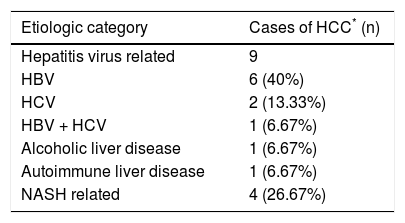
In 15 out of 155 cases of end stage CLD in adults, the CLD was associated with hepatocellular carcinoma (HCC) (Table 4). Eight out of the 15 cases of HCC were treated with trans-arterial chemo-embolisation (TACE) prior to LT. Histopathological examination of these tumors revealed a range of responses, from complete tumor necrosis to completely viable tumor.
Etiologic association with hepatocellular carcinoma.
| Etiologic category | Cases of HCC* (n) |
|---|---|
| Hepatitis virus related | 9 |
| HBV | 6 (40%) |
| HCV | 2 (13.33%) |
| HBV + HCV | 1 (6.67%) |
| Alcoholic liver disease | 1 (6.67%) |
| Autoimmune liver disease | 1 (6.67%) |
| NASH related | 4 (26.67%) |
The distribution of donor biopsies in our series was as follows: 173 intra-operative wedge biopsies from living and cadaveric donors and 30 pre-operative core biopsies. Twelve of the donors underwent both pre-operative core and intra-operative wedge biopsies, 1 donor underwent a core biopsy, followed by a wedge biopsy 2 weeks later, as well as a repeat core biopsy 2 months after that. Two donors underwent repeat core biopsies 20 days and 45 days following their first biopsies.
The acceptable degree of hepatic steatosis for living donor LT has not been firmly defined, but there is a general consensus that macro and/microvesicular steatosis of more than 30% has been associated with a risk of sub-optimal graft function.7 In our program, we have excluded all potential donors revealing steatosis of more than 20%.
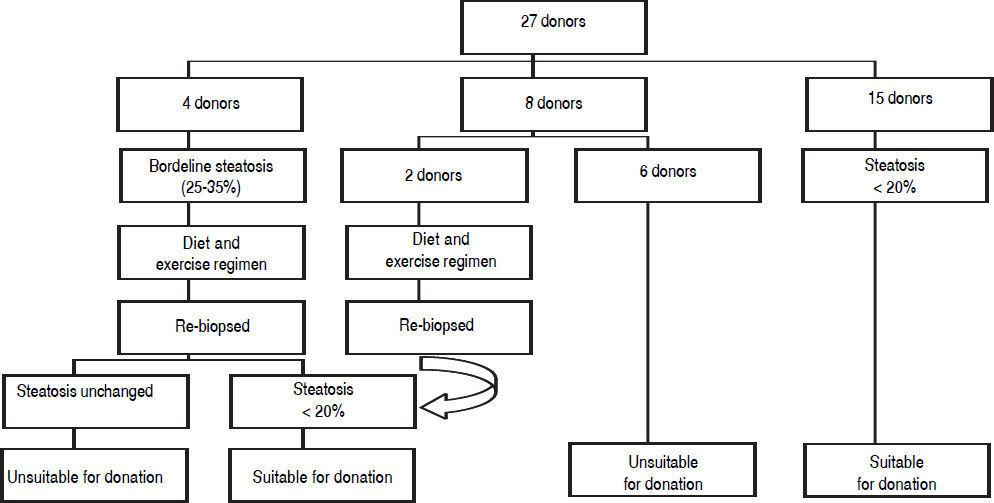
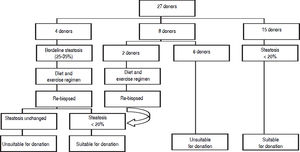
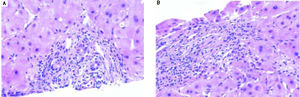
Figure 1 demonstrates the results of the pre-operative core biopsy examination.
The prevalence of mild steatosis (< 30%) was 34.22%, moderate steatosis (30%-60%) was 4.28% and severe steatosis (> 60%) was 1.07% in our series. 60.43% of donor biopsies did not reveal any steatosis.
Incidental epithelioid granulomas were noted in only 5 donor wedge biopsies. These granulomas were non-necrotising, and special stains were carried out to detect acid fast bacilli, fungal organisms and parasites, with negative results.
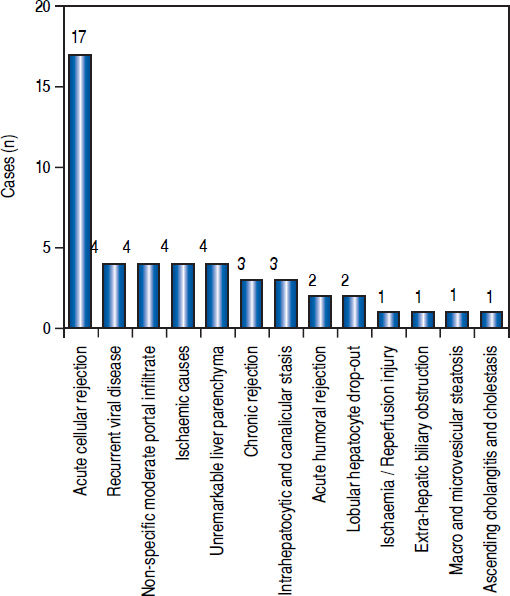
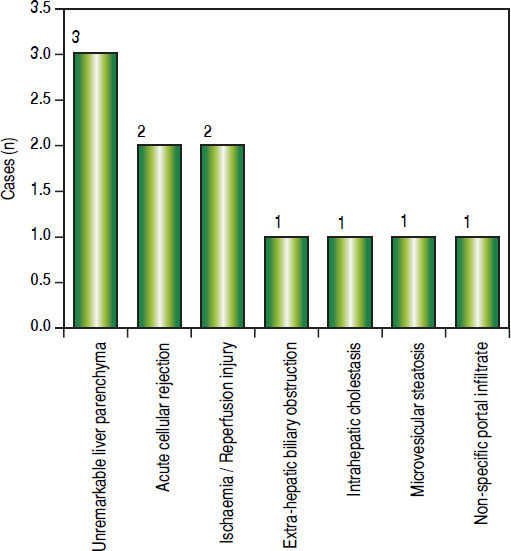
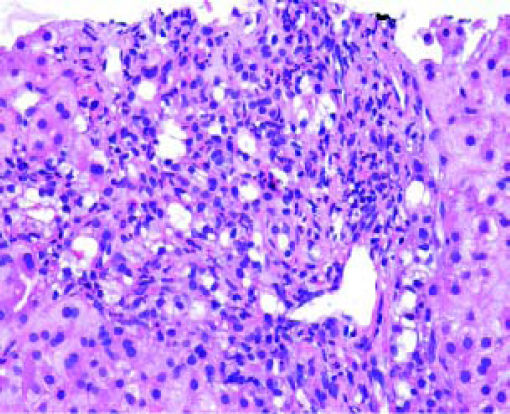
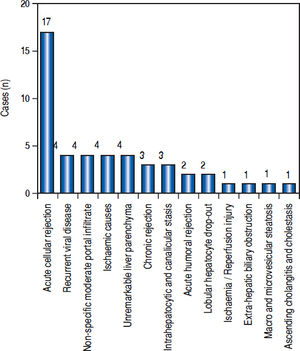
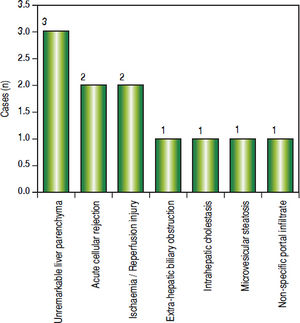
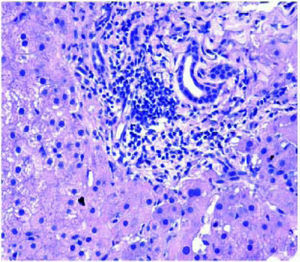
Post-transplant liver biopsiesDuring the study period of 4 years, a total of 58 core biopsies were obtained from 42 patients. Eight of these patients were pediatric patients, who were biopsied a total of 11 times. Seven adults and 3 children were biopsied twice, and 3 adult patients were biopsied 3 times. Acute cellular rejection was the most common diagnosis in adults (Figure 2). In the pediatric patients, the most common diagnosis was essentially unremarkable liver parenchyma, indicating that the rise in liver enzymes and/ bilirubin was not caused by graft dysfunction, but systemic causes (Figure 3).
DiscussionExplant liversEnd stage chronic liver diseaseWe have attempted to analyse the prevalence of the etiologic types of end stage CLD (ESCLD) and acute liver failure (ALF) separately for adults and children ≤ 12 years from a combination of clinical, investigational and representative morphologic data. However, owing to a small sample size and selection of patients from a hospital-based living donor transplant program from a relatively high income group, our data cannot be taken as representative of the prevalence of ESCLD or ALF in the general population of western India. Nevertheless, we believe that as the LT program progresses, we may have a wider representation of patients across the cross-section of the population.
AdultsOut of 155 cases of ESCLD, 47 were due to alcoholic liver disease (ALD). The morphologic features included micronodular and mixed nodular cirrhosis, Mallory hyaline bodies and variable degrees of steatosis. While CLD due to alcohol abuse has long been reported from developed countries, there is a paucity of data on its prevalence in India. A landmark study by Nayak N, et al. (2012) of 372 cases of CLD from North India,8 has put the prevalence of alcohol-related cirrhosis at 23.1%, which is slightly lower than the prevalence in our patients of 30.32%.
Close to a fifth of our ESCLD cases (29 out of 155) were associated with Hepatitis B and C virus (HBV & HCV) infections. While the reported global prevalence of cirrhosis due to Hepatitis virus infections is about 60%,9 the reduced percentage in our study can probably be attributed to a small sample size and lack of a representative patient population from the lower socio-economic strata, in whom the incidence is expected to be higher due to lack of awareness of and inaccessibility to hepatitis B vaccination. However, within the Hepatitis virus group, HCV and HBV are responsible for an equal number of cases of ESCLD, with 1 patient having a co-infection of both viruses. In the developed countries, the stringent implementation of HBV infection control measures including vaccination of all live births, screening of all blood and blood products prior to transfusion, etc have resulted in a reduction in the prevalence of HBV related CLD in recent years.10
The etiology in 12 out of 155 cases was autoimmune liver disease which showed morphologic features of septal lymphoplasmacytic infiltrate and bile ductular proliferation along with serological evidence of autoantibody positivity in all the cases. Non-Alcoholic Fatty Liver Disease (NAFLD) related cirrhosis was diagnosed in 28 cases, after a careful correlation with clinical findings, investigations and the histomorphology which revealed variable grades of inflammation, ductular proliferation and an absence of steatosis. Our prevalence of 18.06% of NAFLD-related cirrhosis is similar to the findings of Nayak, et al., who reported a prevalence of 16.7%.8 Six cases of secondary biliary cirrhosis were seen which included Caroli’s disease and portal cavernoma with extra hepatic portal vein obstruction, 7 cases of cirrhosis were due to metabolic disease which included Wilson’s disease and primary hemochromatosis and 25 cases were classified as cryptogenic since there were no specific morphologic features related to any known entity. Our incidence of 16.13% is similar to the finding of 14.8% cases of cryptogenic cirrhosis reported by Ayata, et al.11
HCC was detected in 15 cases of ESCLD, i.e. 9.68%. The frequency of HCC in other studies based on larger cohorts ranges from 8% to 38%.12 The strong association of HCC with cirrhosis is explained by 2 factors: the repetitive cycles of hepatocyte regeneration provide a milieu for the accumulation of mutations, and secondly the common etiologic agents for cirrhosis, the Hepatitis viruses B and C, play a direct carcinogenic role in the liver.9,13 Two cases were HCV related, 6 were HBV related and 1 was a coinfection of HBV and HCV. Though it doesn’t reflect in our present study, trends show that HCV, rather than HBV is fast becoming a leading cause of HCC, likely due to a rapid increase in blood transfusions, injectibles, intravenous drug abuse and lack of an HCV vaccine.14
Acute liver failureEstablishing the cause of fulminant hepatitis (FH) is an important step in the management of acute liver failure. Fulminant hepatic failure is characterized by a deterioration in liver function and hepatic encephalopathy. The main causes of FH are viral infections, drugs, and indeterminate causes.15 A survey carried out in the United States between 1998 and 2008 in 1147 patients, showed that the major etiologies of ALF were paracetamol overdose (46%) followed by indeterminate causes (14%), drug-related (11%), HBV (7%), other causes (7%), autoimmune hepatitis (5%), ischemic hepatitis (4%), hepatitis A virus (3%), and Wilson’s disease (2%).16 From the European Liver Transplantation Registry, the most common causes of transplanted FH between 1972 and 2007 were viral causes (HAV: 1%; HBV: 15%), paracetamol overdose (8%), non-paracetamol drug overdose (antibiotics > ecstasy > anti-tubercular treatment > Non-steroidal anti-inflammatory drugs) (11%), indeterminate causes (48%), and other causes (17%).15 In our series, the etiologies of ALF leading to LT were: anti-tubercular treatment (ATT) related (27.27%), hepatitis E virus (HEV) related (27.27%) and vascular/ischaemic related (18.18%), indeterminate (9.09%), Rattol poisoning related (9.09%) and due to extensive metastatic malignancy (9.09%).
However, despite intensive investigations and the use of the most modern techniques [polymerase chain reaction (PCR) methods, molecular techniques, genomic DNA, and biochemical methods], the causes of most cases of FH or ALF may remain unknown.15
Acute liver failure secondary to ATT is rare in the developed world. These cases represented only 2.47% of the total number of patients admitted with ALF between 1986 and 2008 in a study from France.17 In a retrospective study of patients from the United Network for Organ Sharing registry (1987-2006), only 0.07% of LT procedures for ALF were due to ATT (50 of 73,977 transplant patients).18 However, tuberculosis (TB) continues to remain a significant infectious disease across much of the developing world. ATT is a leading cause of drug-induced liver injury (DILI) in India and of drug-induced acute liver failure leading to death (DI-ALF).19 In contrast, non-TB antibiotics and paracetamol are the commonest causes of DILI and drug-induced ALF in western countries. In a large series of acute liver failure cases in New Delhi, anti-TB drugs contributed to 5.7% patients with ALF(70/ 1223), with 67% mortality.20–22
HEV is an enterically transmitted RNA virus responsible for large waterborne epidemics of ALF or FH in endemic regions such as India, Central America, Africa, and Asia.23 The overall mortality due to fulminant liver failure in endemic regions varies from 0.5% to 4%, but in pregnant women with HEV FH, mortality is higher (20%). Our patients were females who contracted the infection during the second half of their pregnancies.
Children ≤ 12 yearsESCLDBiliary atresia is the most common cause of chronic cholestasis and LT in children, with the mean age of LT for patients being 11 months.24,25 Out of 11 children with ESCLD, 3 patients i.e. 27.27% had secondary biliary cirrhosis due to biliary atresia- 2 females and 1 male, ranging in age from 8 months to 7 years at the time of LT. In 2 of the older patients within the cohort, i.e. 11 and 12 years, the etiology was autoimmune, which is in keeping with the common causes of ESCLD in older children and adolescents.24
In 3 children it was not possible to ascertain the cause of cirrhosis and hence were labelled cryptogenic. Cryptogenic cirrhosis in children is hypothesised to result from the progression of fatty liver disease or from the effects of complex metabolic syndromes, such as mitochondriopathies.26
The etiology in 2 of the children was Wilson’s disease, and both patients had elevated levels of dry weight of copper in the explant livers.
One patient presented with hepatopulmonary syndrome in the setting of an Abernathy malformation, and focal nodular hyperplasia was incidentally noted within the explant liver.
Acute liver failureIn a large prospective study in Europe and North America, by the Pediatric Acute Liver Failure Study Group, in 49% of patients, the cause of ALF could not be determined.27 In our admittedly small series also, we were unable to ascertain the cause of liver failure in 2 pediatric patients, who did not show elevated titres of viral markers, had negative blood cultures and had no history of intake of medication.
Donor biopsiesThe new millennium has seen a rapid rise in the prevalence of obesity, diabetes mellitus and the metabolic syndrome.28 The sequelae of these states on the liver is the development of NAFLD, with deposition of triglycerides in the liver.29 In the scenario of liver transplantation, severe steatosis i.e. > 60%, of the donor liver is a risk factor for primary non-function of the engrafted liver, while moderate steatosis i.e. 30%-60% has also been associated with a risk of suboptimal graft function.7,29
Although many LT programs advocate protocol liver core biopsy as part of donor evaluation, in our centre biopsies have been performed selectively for the evaluation of steatosis when an imaging modality shows findings of increased fat content in the liver or the BMI exceeds 30.
The prevalence of moderate steatosis in our series of both living and cadaveric donors is 4.28%. After excluding the 28 cadaveric donors, the prevalence of moderate steatosis in living donors is 4.40%, which is significantly lower than the value of 25% reported by Selzner, et al. in the living donor population in the West.30 This lower prevalence may be attributed to the lower mean age of the living liver donor population i.e. 36.20 years and a preponderance of women i.e. 93 women compared to 60 men.
The presence of incidental granulomas in donor biopsies may have a large number of etiologic possibilities, including infective, drug-induced and autoimmune, to name a few.31 In the Indian scenario, TB continues to be the leading culprit of granulomatous inflammation.19 In such an eventuality of proven tubercular infection in the donor, the immunocompromised recipient should be closely monitored for any manifestation of active tubercular infection. The decision to institute first line ATT will then have to be weighed against the risk of their hepatotoxic effects on the transplanted liver.
In our study, the recipients were closely followed up, but did not manifest any signs of active TB. The donors also did not develop signs or symptoms of active pulmonary or extra-pulmonary tubercular disease.
Post-transplant liver biopsiesLiver allograft pathology plays a crucial role in the diagnosis and management of patients post LT. Liver needle biopsy is a convenient method for monitoring allograft function. Interpretation of post-transplant biopsies requires knowledge of the original disease, time after transplantation and the immunosuppressive drug levels. The histopathological features of acute and chronic rejection are well established and liver biopsy continues to be the “gold standard” for diagnosing these two conditions.
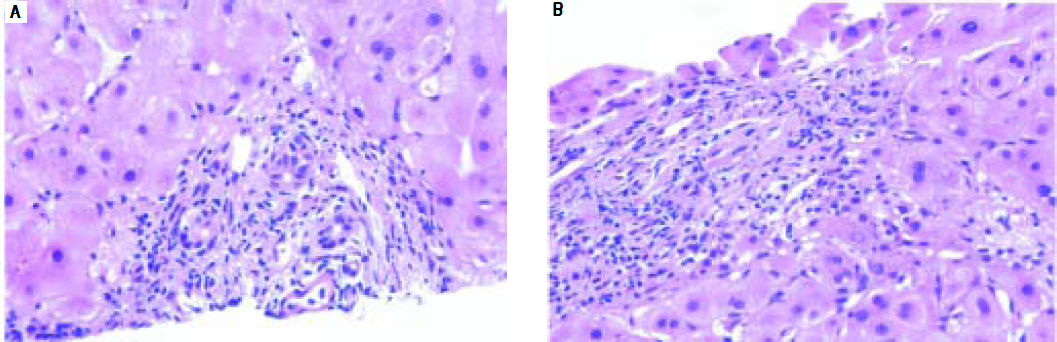
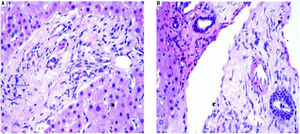
AdultsIn our study, acute cellular rejection (ACR) was the most common finding in post-transplant biopsies, occurring in 12 patients, 2 of whom were biopsied on 3 separate occasions, and 3 of whom were biopsied on 2 separate occasions. One of the patients who was biopsied thrice had moderate, severe and mild acute rejection in sequential biopsies carried out on days 55, 143 and 390 post-transplant (Figures 4 and 5); while the other patient to be biopsied thrice, had mild, moderate and mild rejection in sequential biopsies carried out on days 503, 796 and 820 post-transplant. Among the 3 patients who were biopsied twice each, 2 were reported as ACR in one of their 2 biopsies, with the second biopsies showing non-specific portal in-flammation. The 3rd patient had severe ACR in the 1st biopsy and mild ACR in the 2nd biopsy. The commonest cause for most of these episodes of rejection was non-compliance with immunosuppressant drugs. ACR was also the commonest finding in larger series of post-transplant liver biopsies published from Iran and China.32,33
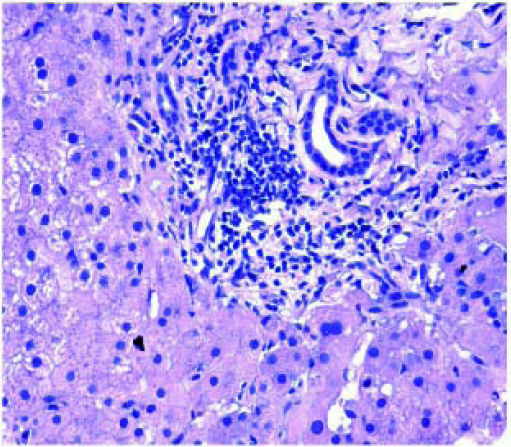
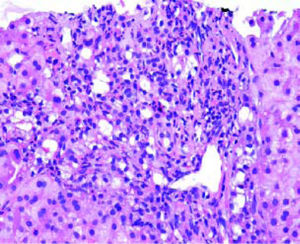
The next major histopathologic findings were recurrence of hepatitis C in the allograft liver which was noted in 3 patients (Figure 6) Re-infection of the liver allograft with the hepatitis C virus after transplantation is practically a universal occurrence.34,35 Hepatocyte expression of HCV antigens has been demonstrated as early as 10 days after LT.36 In our study, the post-transplant biopsies were performed between 87 to 207 days post LT. Two of the biopsies showed early changes of recurrence i.e. lymphocytic infiltrate in the portal tract associated with lobular inflammation. The other 2 biopsies showed chronic changes of reccurrence with METAVIR grades for fibrosis ranging from 2-3.37 All the cases had high serum viral loads. Our findings reflect those in other studies which show that acute hepatitis affects the graft between 2-5 months post LT while chronic hepatitis is established about 6-12 months post LT.38
We reported 4 biopsies as non-specific moderate portal infiltrates without evidence of rejection, accompanied by cholestasis, which did not warrant any active intervention, and 4 biopsies as unremarkable liver parenchyma. The indications for biopsy in these patients were elevated liver enzymes and/ bilirubin levels, which were probably secondary to systemic infections and underlying co-morbidities.
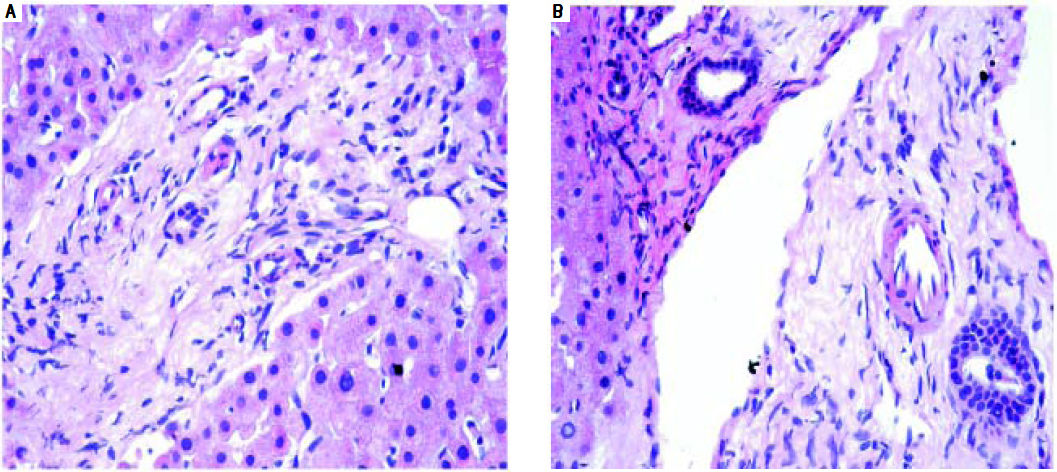
The incidence of chronic rejection has been reported to be between 2 and 20% in literature.32 We have reported 3 biopsies as chronic rejection during our study period (Figure 7).
Three patients had extensive intrahepatocytic and canalicular bile stasis, and were found to have biliary strictures on imaging. Four patients showed sinusoidal and pericentral zone congestion and hemorrhage, probably secondary to ischaemic causes.
One patient who had uncontrolled diabetes mellitus and had been transplanted for NASH-related CLD, showed macro-vesicular steatosis involving about 20% hepatocytes. The biopsy from the lone patient transplanted for Caroli’s disease showed ascending cholangitis, cholestasis and portal fibrosis in a biopsy taken 1002 days post-transplant.
Two biopsies showed lobular hepatocyte dropout, without any accompanying inflammation or collapse of the reticulin framework, which could not be explained, even after extensive investigations.
We had 1 case of reperfusion injury, 2 cases of acute humoral rejection and 1 case of extrahepatic biliary obstruction (Figure 8), which were lower than the documented percentages in other studies.32,33 We have not reported any cases of cytomegalovirus infection, post-transplant lymphoproliferative disorder or post liver transplant cirrhosis in the present series.
Children < 12 yearsAmong the pediatric patients who underwent LT, 8 patients were biopsied for rising liver enzymes and / serum bilirubin levels (Figure 3). ACR was seen in 2 patients aged 11 years and 19 months (at the time of biopsy), on days 518 and 53 post transplant respectively. The ACR was of a severe grade in the former, and of a mild grade in the latter. This low rate of ACR in our series is similar to the findings of Shepherd et al, who studied more than 2,000 pediatric patients < 18 years who have undergone LT during an 11 year period, and reported the overall rejection rate as 0.29 episodes per patient year (0.20 in infants and 0.44 in adolescents).39
The biopsies of 2 patients revealed changes of ischaemia/reperfusion injury. The biopsies were obtained on days 16 and 15 post transplant respectively, and both recipients underwent reduced left lateral segment living donor liver transplants.
A biopsy from a 12 year old patient on day 39 post transplant revealed features of extra hepatic biliary obstruction. Two biopsies were performed on a 10 year old patient who was transplanted for Wilson’s disease and presented with high grade fever and abdominal pain, on days 115 and 147 post transplant. The 1st biopsy revealed non-specific portal inflammation and sinusoidal congestion, and the patient was started on antibiotics and appeared to improve. However the patient was re-admitted 20 days following discharge, with deranged liver enzymes, and the biopsy revealed cholestatic liver injury and spotty necrosis. Biliary complications occur in approximately 10%-30% of pediatric liver transplant recipients40 and is noted in 2 biopsies in our series over a 4 year duration.
Two patients were biopsied after presenting with high grade fever, decreased appetite and slightly elevated liver enzymes, to rule out rejection or cytomegalovirus infection. The viral loads were within normal limits and the biopsies did not reveal any abnormalities. One of these patients, a 3 and a half year old male, again presented with symptoms of acute gastroenteritis and elevated liver enzymes, about 200 days following his prior admission, and was re-biopsied. His biopsy revealed prominent micro-vesicular steatosis, following which the administration of Methylprednisolone was stopped.
We have not reported any cases of post-transplant viral or fungal infection, post-transplant lymphoproliferative disorders, recurrent or de novo autoimmune hepatitis or chronic rejection necessitating re-transplantation among our cohort of pediatric recipients.
Conclusions- •
In our series of explant hepatectomies in adults, alcoholand hepatitis- virus related CLD are leading indications for liver transplantation. HCV, more than HBV, is now the major cause of both CLD and HCC.
- •
Biliary atresia is the most common cause of ESCLD and LT in children, which is mirrored in the results of our study.
- •
Steatosis in donor livers is a risk factor for inferior graft function after orthotopic liver transplantation. The prevalence of 4.28% of moderate steatosis (between 30-60% steatosis) in our series is much lower than that documented in western literature.
- •
The finding of only 5 cases of non caseating granulomas in our case series of donors was unusual for a country like India, which has the highest world-wide burden of tuberculosis.
- •
In our analysis of post-transplant liver biopsies in adults, the most common pathological diagnoses have been acute cellular rejection, followed by recurrence of the primary viral disease and non-specific moderate portal inflammation. Early and accurate diagnosis aids the clinicians in titrating the dosage of immunosuppressants and anti-viral medications, thus optimising graft function.
- •
Among the pediatric cohort, the most common diagnoses have been essentially unremarkable liver parenchyma due to non-hepatic etiologies, followed by ACR and ischaemia/reperfusion injury.
- •
As we share our experience 4 years into our LT programme, we expect that our data on explant hepatectomies, donor biopsies and post-transplant liver biopsies will more closely mirror the global trends in the years to come.
- •
ACR: acute cellular rejection.
- •
ALD: alcoholic liver disease.
- •
ALF: acute liver failure.
- •
BMI: body mass index.
- •
CLD: chronic liver disease.
- •
CT: computerised tomography.
- •
ESCLD: end stage chronic liver disease.
- •
FH: fulminant hepatitis.
- •
H & E: haematoxylin and eosin.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HEV: hepatitis E virus.
- •
HIV: human immunodeficiency virus.
- •
LAI: liver attenuation index.
- •
LT: liver transplantation.
- •
NAFLD: non alcoholic fatty liver disease.
- •
PCR: polymerase chain reaction.
- •
TACE: trans:arterial chemo:embolization.