There is a shortage of ideal donor organs with consequential increasing waitlist times, drop-off, and mortality. Teams have thus extended the donor criteria. Little is known about patients’ actual choices and what factors may influence their decisions regarding different extended criteria liver grafts.
Patients and MethodsThe documented acceptance or refusal of seven extended criteria liver graft types of patients consented for transplant in a single institution over a 2-year period was reviewed. Patient factors including sex, age, indication, aetiology, and model for end-stage liver disease (MELD) score were analysed using logistic regression.
ResultsMost patients were willing to accept most graft types. MELD score did not impact the acceptance or refusal of any graft type. Older patients and those with hepatocellular carcinoma (HCC) or ascites had significantly higher rates of acceptance. Hepatitis B or C disease aetiology was predictive of willingness to accept a similarly infected graft, respectively. HCC was predictive of acceptance of grafts from donors with a cancer history.
ConclusionsIn general, patients embrace the available extended criteria donors. Our analysis suggests that consent should be revisited as patients deteriorate or ameliorate on the waitlist, especially if in the form of ascites or HCC but not necessarily MELD score.
Brain death was defined and promoted by the Committee of the Harvard Medical School in 1968 [1]. Although since updated [2], this standardisation enabled the widespread donation of organs for transplant from such patients. Donation after brain death (DBD) has become the most commonly used graft in the context of liver transplant [3–5]. There is however a paucity of such organs. Waiting list numbers exceed that which is available, contributing to increasing waitlist times, waitlist drop-off, and waitlist mortality [6, 7]. This led to efforts to expand the donor organ pool, utilising so called marginal or expanded-criteria grafts. Such grafts include organs from donors after circulatory death (DCD), split liver donations, donors with a history of cancer, of hepatitis B (HBV) or C (HCV) infection, or behaviours which put them at risk of such and related infections. Living related donor transplant is also being utilised in many institutions.
Each of these graft types are associated with different risks. DCD grafts are associated with an increased risk of ischaemic cholangiopathy, and up to 2-fold greater risk of graft loss and mortality when compared with DBD donors [8, 9]. Split liver grafts have not been found to lead to any difference in mortality or graft loss when compared with DBD donors but are associated with an increased risk of perioperative biliary and vascular complications [10, 11]. Receiving an organ from a donor with a history of cancer carries with it the potential for inadvertent transmission. The term cancer however comprises a heterogenous cohort of diseases. The Council of Europe has published an extensive guide on the transmission risks of various cancers at transplant ranging from minimal (e.g. basal cell carcinoma of the skin) to unacceptable risk (e.g. Kaposi's sarcoma). In general, complete remission of 5–10 years is recommended before consideration of cancer donors, although grafts from patients with certain active primary CNS malignancies are used [12]. There is no difference in graft or overall survival for recipients of HBV core antibody positive donor grafts, although antiviral prophylaxis is required [13]. HCV too is a treatable disease, and most patients can expect cure. No difference in graft or overall survival has been found in this context [14, 15].
At our institution, following informed consent, patients advise their willingness to accept different donor types. Before being placed on the transplant waiting list, they meet with the surgical team as the final part of informed consent. At this time, they may consent to receive DCD grafts, split liver grafts, livers from donors with a history of cancer, of HBV or HCV infection, or increased risk donors (IRD). IRDs refer to donors whose behaviours put them at risk of transmissible viral infections, such as intravenous drug use. Prior to this discussion they will have received a standard proforma detailing information on the various graft types. In this work, we collated the consent forms of patients placed on the liver transplant waiting list over a 2-year period and analysed patient characteristics that influenced the acceptance or refusal of extended criteria grafts. We believe this is the first such study assessing the actionable decisions of patients awaiting liver transplant, as opposed to hypothetical surveys.
2Methods2.1Study populationAll patients who were consented for liver transplant between January 2016 and December 2018 were included. Consent for subtypes of extended criteria grafts was obtained at the time of activation on the transplant waiting list. Consent forms were reviewed, and the relevant data collated. All demographic data was taken from presentations made on patients at the multidisciplinary team meeting where they were deemed appropriate for and in need of a liver transplant. Patients may have one or more disease aetiologies and may have one or more indications for liver transplant. This work was registered with and approved via the local ethical protocols and conformed to the ethical guidelines of the 1975 Declaration of Helsinki. No organs from executed prisoners were used.
2.2Statistical analysisThe statistical analysis was performed by a statistician (B.J.) who was independent of the clinical research team. All data analysis was performed using R (v. 3.6.3). For each of the 7 liver types, a binary logistic regression model was derived to predict the acceptance (or refusal) by each patient, given information about 15 possible explanatory variables. The optimal model was chosen by a probabilistic model selection algorithm based on the Akaike Information Criterion (AIC) method. This method was chosen to avoid statistical overfitting, as explanatory variables are only included when the increase in the predictive power is sufficiently large. The AIC is a number which quantifies the predictive power of the model but adds a penalty for each additional explanatory variable. Thus, the model with the best (lowest) AIC is the most parsimonious model. Further information is provided in supplementary material.
The validity of the chosen model was verified by computing the p-value for the null hypothesis that the chosen model performed no better than a model without any of the explanatory variables. Significance was determined using the Bonferroni correction for a significance threshold of 0.05 with 7 individual tests, i.e. p-value <0.0071. The pseudo-R2 value was used for additional validation.
A selection of pseudo-R2 values is also provided in the results. The reader should not conflate pseudo-R2 values (used here in the case of logistic regression) with R2 values (used in simple linear regression). Pseudo-R2 values represent an attempt to create an R2 statistic for logistic regression. However, because the outcome variable in logistic regression is binary (e.g. acceptance or refusal), there is disagreement on the best way to calculate this (hence there are several approaches, of which we have chosen three). Secondly, there is no pseudo-R2 cut-off above which can be deemed “acceptable”. Their usefulness thus lies in enabling the reader to compare the explanatory power of the models.
A sensitivity analysis was performed as some arbitrary choices have necessarily been made to narrow down the optimal model to one. The first parameter was the stepwise “direction” of the AIC algorithm: our arbitrary choice was to start with a “null” model (i.e. model with zero explanatory variables) and add and subtract a single variable until the best model (with the lowest AIC) was reached. However, there are three alternatives: full-to-null, null-to-full with variable addition only, and full-to-null with variable subtraction only. The second parameter was the handling of laboratory model for end-stage liver disease (MELD) scores for patients on anticoagulation. MELD score cannot be calculated for patients on anticoagulation, so we imputed the missing values using the median MELD scores. For the sensitivity analysis, we re-ran the analysis without the patients on anticoagulation (4).
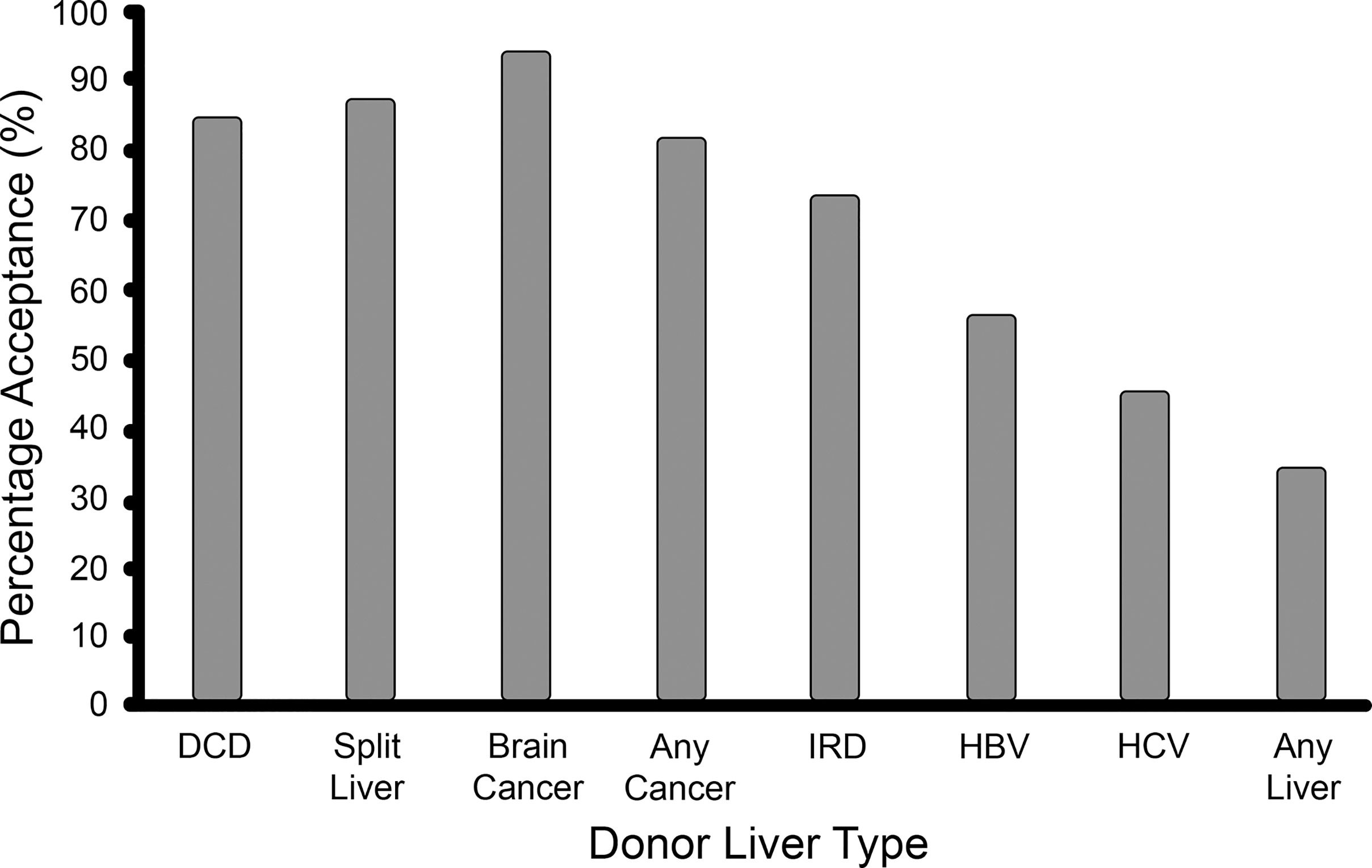
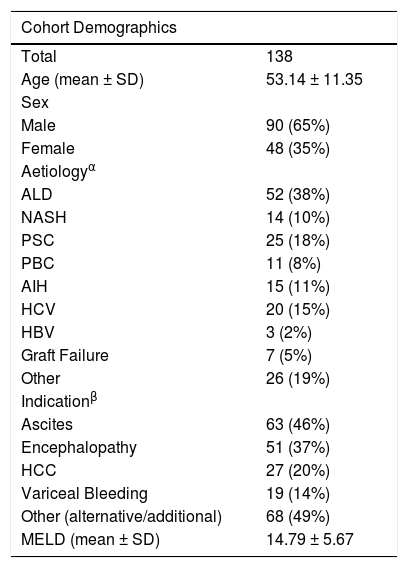
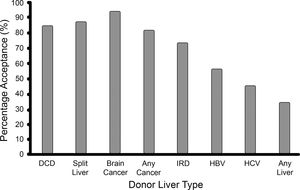
3Results3.1Cohort characteristicsIn this study, 138 patients were included. Their demographic details are listed in Table 1. It was a predominantly male cohort (65%), with a mean age of 53 years, and a mean laboratory MELD score of 15. While most patients were willing to accept most graft types, only one third of the cohort were willing to accept any graft type (see Fig. 1). Ascites, encephalopathy, and hepatocellular carcinoma (HCC) were the most common clinical indications while alcohol related liver disease (ALD), primary sclerosing cholangitis (PSC), and HCV infection were the most common disease aetiologies.
Demographics of the patient cohort. APatients may have multiple aetiologies. BPatients may have multiple indications.
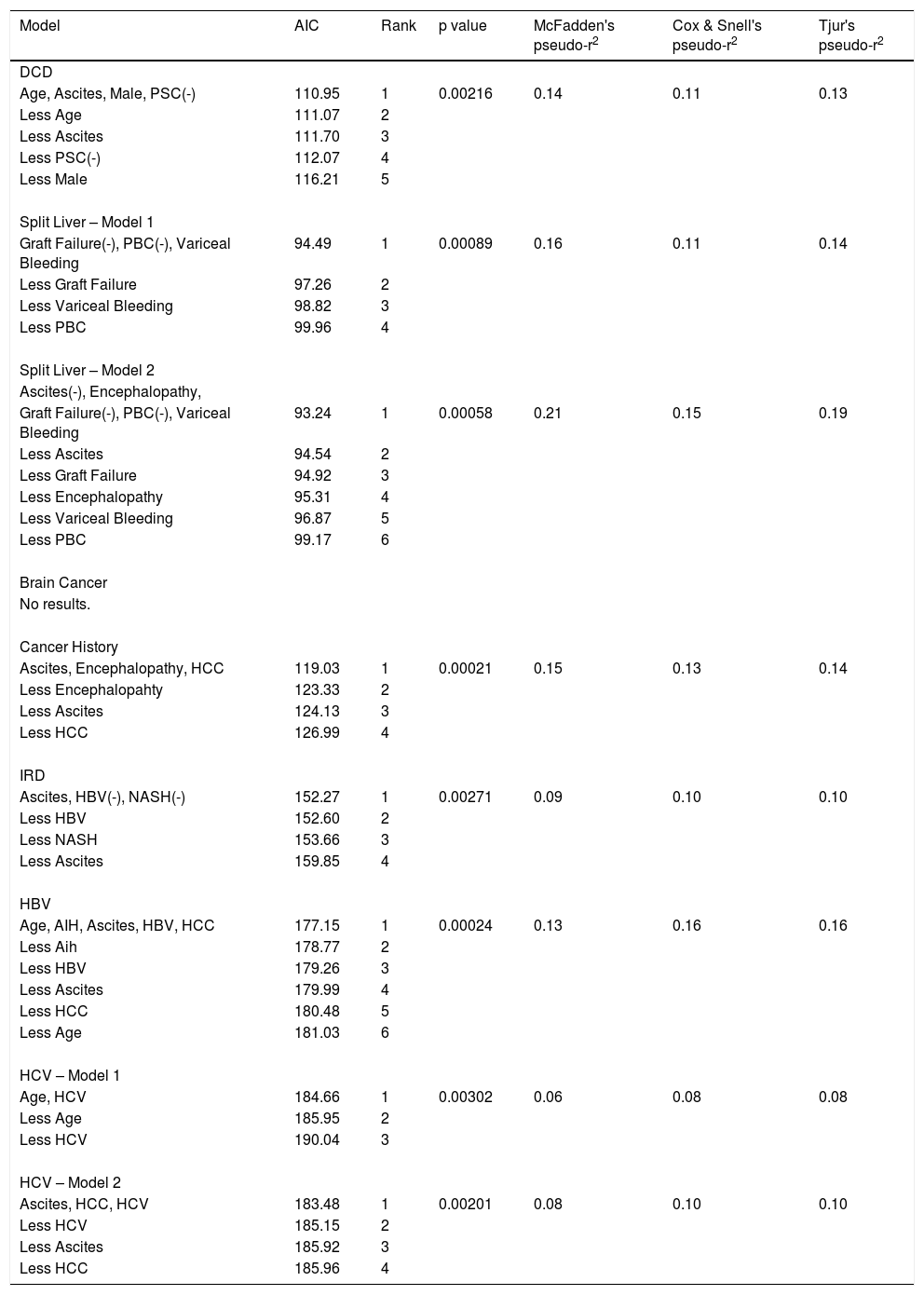
Eighty-five percent of patients consented to receive a DCD graft. The chosen model comprised four explanatory variables (p = 0.00216, pseudo-R2 range: 0.11–0.14). No alternative models were identified by the sensitivity analysis. Increasing age, ascites, and male sex were independently associated with acceptance of DCD grafts, while PSC was associated with refusal. See Table 2.
AIC model results for each graft type. (-) indicates refusal.
Eighty-eight percent of patients consented to receive a split liver graft. Two models were identified, both containing the same three variables: variceal bleeding increased acceptance while graft failure and primary biliary cholangitis (PBC) decreased acceptance. Model 1 (p = 0.00089, pseudo-R2 range: 0.11–0.16) was identified via the default model selection pathway and contains just these three variables, whereas Model 2 (p = 0.00058, pseudo-R2 range: 0.15–0.21) was identified via the full-to-null pathway and contains two extra variables: encephalopathy and ascites. However, on sense-checking these models, it was noted that all 19 patients with variceal bleeding accepted a split liver graft. This is clinically relevant, but statistically, it has the effect of making the logistic regression model unstable, and more susceptible to overfitting and less robust to minor changes in the data. Accordingly, the less parsimonious Model 2 was not robust to the removal of the anticoagulated patients, suggesting that the two additional variables may be the result of overfitting. Conversely, in support of Model 1, the only variables to feature in all models identified via the sensitivity analysis (both with the variceal bleeding variable included and excluded) are the three variables of Model 1. Hence, we can confidently conclude that variceal bleeding, PBC and graft failure are robust predictors of acceptance/refusal of a split liver graft, while acknowledging that other variables may play a role. See Table 2.
3.4Donor with current brain cancerNinety-four percent of patients consented to receive a graft from a donor with brain cancer, i.e. only 8 patients out of 138 refused. This finding is of clinical relevance, however from a statistical perspective, the resulting dataset is highly imbalanced. In essence, there are only 8 informative datapoints. Given that there are 15 potential explanatory variables, and 8 effective datapoints, any proposed model would be exceptionally vulnerable to overfitting. The AIC-based model selection algorithm did output a parsimonious model with two variables, robust to the sensitivity analysis. However, in accordance with the above concerns, this model exhibited clear evidence of overfitting with near-infinite parameter estimates (i.e. an odds ratio for acceptance of 81 million and 49 million for ALD and variceal bleeding, respectively). It may be of clinical interest that all 52 ALD patients and all 19 variceal bleed patients accepted a graft from a donor with brain cancer, but the same is nearly true of all 15 explanatory variables and simply reflects the high acceptance rates for this graft type in the cohort as a whole. Hence, we can conclude nothing beyond a high acceptance rate of grafts from a donor with brain cancer generally. See Table 2.
3.5Donor with a past history of cancerEighty-two percent of patients consented to receive a graft from a donor with a past history of cancer. The chosen model comprised three explanatory variables (p = 0.00021, pseudo-R2 range: 0.13–0.15). No alternative models were identified by the sensitivity analysis. Ascites, encephalopathy, and HCC were associated with acceptance of grafts from donors with a past history of cancer. See Table 2.
3.6IRD graftSeventy-four percent of patients consented to receive an IRD graft. The chosen model comprised three explanatory variables (p = 0.00271, pseudo-R2 range: 0.09–0.10). No alternative models were identified by the sensitivity analysis. Ascites was associated with acceptance while non-alcoholic steatohepatitis (NASH) and HBV were associated with refusal of IRD grafts. See Table 2.
3.7HBV positive donor graftFifty-seven percent of patients consented to receive an HBV core antibody positive graft. The chosen model comprised five explanatory variables (p = 0.00024, pseudo-R2 range: 0.13–0.16). No alternative models were identified by the sensitivity analysis. Increasing age, autoimmune hepatitis (AIH), ascites, HBV, and HCC were associated with acceptance of HBV positive donor grafts. On sense-checking the chosen model it was noted that all HBV patients accepted an HBV positive graft. This did not have any of the disruptive effects seen in the case of the split liver grafts. Accordingly, when the HBV variable was removed, the resultant model, although inferior, contained the other four variables and was robust to all changes made in the sensitivity analysis. See Table 2.
3.8HCV positive donor graftForty-six percent of patients consented to receive an HCV positive donor graft. Several models were identified following the sensitivity analysis, all of which contained the HCV variable. Two were considered valid. The remainder had at least six variables and were not robust to the sensitivity analysis, suggestive of overfitting. Model 1 (p = 0.00302, pseudo-R2 range: 0.06–0.08) was identified by the default model selection pathway and contained two variables: increasing age and HCV infection increased acceptance of HCV positive grafts. Model 2 (p = 0.00201, pseudo-R2 range: 0.08–0.10) had three variables: HCV infection, ascites and HCC increased acceptance. In conclusion, we can be confident that HCV infection predicts increased acceptance of HCV positive grafts, while the data suggests that increasing age, ascites and HCC also play a role. See Table 2.
4DiscussionExtended criteria liver grafts are the transplant community's answer to the shortage of available optimal DBD organs. This expansion of the donor pool is effective. It has been shown to reduce waitlist mortality [16]. As outlined, it does however carry both risks and costs. While clinicians driving these efforts seem satisfied to tolerate them, the available data on what patients consider acceptable when consenting for liver transplant and of what patient factors influence such decisions is derived from surveys. In this work we analysed their actionable decisions at the time of consent for liver transplant, as opposed to considering hypothetical scenarios.
Surveys of liver transplant candidates in America and Brazil have found a high willingness to accept split liver grafts, 90% and 91% respectively, even when told it may lead to a reduced relative survival [17, 18]. This is similar to the attitudes of transplant candidates here, where 88% of candidates consented to receive a split liver graft. The willingness to accept an IRD graft is higher in this Irish cohort than described elsewhere. 74% of patients here were willing to accept such a graft. A survey of liver transplant candidates in Canada found that only 41% of respondents would consent to this but rose to 63% with further education of the survey participants on the facts surrounding IRD donors. They do hypothesise that their results would be higher if the answers were applicable, as here [19]. The willingness to accept confirmed infected grafts is lower amongst liver transplant candidates here by comparison to that for potentially infected grafts; 54% of patients consented to receive an HBV positive graft, and 46% an HCV positive graft. It is unclear why HCV positive grafts were the only graft type to have an acceptance rate of less than 50% in this cohort, especially in the post direct-acting antiviral era where the trend has been a welcoming of such grafts by liver transplant candidates [20]. It may be related to the controversy surrounding the state infection of many patients with HCV up to the 1990s in Ireland through the administration of contaminated blood products. Further education of patients regarding HBV and HCV may influence these acceptance rates, as aforementioned for IRD grafts [19]. The risk of cancer was not an insurmountable obstacle to consent for extended criteria donor grafts in this cohort. 94% were willing to accept a graft from a donor with current brain cancer, and 82% a graft from a donor in remission with a past history of cancer. Taken together, these figures reveal a high willingness amongst liver transplant candidates to accept many of the risks and costs associated with extended criteria liver grafts and would appear to support the clinician efforts to expand the donor pool. These findings are more nuanced but in line with previous reports suggesting a high willingness amongst liver transplant patients to accept extended criteria grafts [21, 22].
Another aim of this work was to determine which, if any, disease related factors significantly influenced patient decision making regarding the acceptance or refusal of extended criteria liver grafts. There is little published data available on this. The aforementioned survey of Canadian liver transplant candidates found that women were more likely than men to accept an IRD graft [19]. We found no such relationship with sex for IRD grafts. Ascites was associated with acceptance while NASH and HBV disease aetiologies were predictive of refusal of such grafts. Male sex was however found to positively influence the acceptance of DCD grafts. This is interesting as DCD grafts are shown to lead to reduced graft and overall survival by comparison to DBD grafts [8, 9]. These findings differ with previous work which found that men awaiting liver transplant tended to be less risk tolerant than women [21].
A second interesting result in the DCD model was that PSC aetiology predicted refusal of such grafts. PSC is a stricturing disease of the biliary tree and may have a similar phenotype to post-transplant ischaemic cholangiopathy which is a known high-risk complication of DCD grafts. This finding varies from the trends in other results which suggest that patients are happy to accept the risks of some similar (potential or definite) complications of their current disease in their transplanted graft. The presence of HCC was predictive of acceptance of grafts from those with a history of cancer. Recipient HCV infection was found to be predictive of the acceptance of an HCV positive graft in both HCV models. Recipient HBV infection was predictive of acceptance of HBV core antibody positive grafts. This willingness may relate to the similarity-attraction effect; the finding that perceived likeness to others stemming from a shared comparable (health) experience is associated with appeal for one's counterpart [23].
Ascites as an indication for transplant was found repeatedly to be predictive of acceptance of extended criteria grafts. It was found to be significantly associated with the acceptance of DCD grafts, HBV positive grafts, HCV positive grafts, IRD grafts, and grafts from donors with a history of cancer. Rodrigue et al. have previously found ascites to be predictive of acceptance of higher mortality risk post-transplant in a survey of liver transplant candidates [22]. Both HCC and increasing age also featured frequently amongst the results. HCC as an indication for transplant was found to be associated with the acceptance of HBV positive grafts, HCV positive grafts, and grafts from those with a history of cancer. Increasing age was predictive of acceptance of DCD grafts, HBV grafts, and HCV grafts. The reasons behind these results are unclear. It could be due to poor health related quality of life. Ascites, HCC, and increasing age have all been shown to negatively impact health related quality of life in liver disease [24–27]. This is true too for encephalopathy [24, 25]. Consistent with this encephalopathy was found to be predictive of acceptance of grafts from donors with a history of cancer. Our results suggest that decompensation particularly with ascites, the development of HCC, or older age at the time of consent, pressures patients towards the acceptance of risks and costs that they may otherwise not have tolerated. These are important insights for clinicians considering the welfare of patients being considered for liver transplant, particularly in the context of MELD exception indications.
MELD score is used as an objective cut-off for transplant mortality benefit [28], but did not emerge as a significant factor for the acceptance or refusal of any extended criteria liver graft. Previous reports have conflicted on its utility in predicting patients’ attitudes towards such grafts [21, 22], and have shown MELD score to not be predictive of health-related quality of life [24, 25]. Clinical decompensation with ascites, the presence of HCC, or older age at the time of consent appear to be more important than disease severity as measured by MELD when deciding on the tolerable risks and costs associated with extended criteria grafts for patients.
This work has several limitations. Although extensive, the list of graft types here is not exhaustive. Living related donor transplant is not performed here, and so is not included in this analysis. This work has focused primarily on disease related factors influencing the decision-making process. There may be other factors involved for which we have not accounted. Race has previously been shown to influence the acceptance of extended criteria grafts and tolerability of higher post-transplant mortality in a survey of liver transplant candidates [22]. Factors such as religion, beliefs, and education have not been accounted for here. The beliefs of the surgeon at the time of consent may also play a role in the patient decision making process despite the use of standard written proformas.
An exploratory data analysis approach based on logistic regression was taken over a confirmatory data analysis approach given the absence of published data on actionable patient decisions at the time of listing for liver transplant. This technique allows us to model the probability of accepting a particular liver as a function of patient characteristics, from a dataset which records only acceptance or refusal, and accommodates for the non-linear relationship between variables. A confirmatory analysis would be preferable given it lessens the probability of experiencing type 1 errors. However, confirmatory analysis is only possible when a limited set of predetermined hypotheses exist. Surveys of hypothetical scenarios, while interesting, were deemed insufficient basis for a confirmatory analysis in particular when the authors of such work surmise that the answers may be different in the context of actionable decisions, as here [19]. Our model outputs are considered independent from one another and while odds ratios were found, given the exploratory nature of the work, only positive and negative signs were interpreted. Not all significant models were stable. Multiple significant models were found for both HCV and split liver grafts, belying underlying instability within these models. These results are thus interpreted with caution. For brain cancer donors no logistic regression results were found. This is a function of the high rate of acceptance of this graft type amongst the cohort (94%). As with all such forms of regression analysis, multicollinearity prohibits our ability to make unequivocal statements about the relationship between the explanatory variables and graft acceptance.
Surveys have shown that patients would like to play a role in the decision-making process regarding the acceptance or refusal of different graft types [21, 29]. As outlined, yet more surveys have attempted to elucidate which patient factors may affect such decisions. Ours is not a hypothetical attitude survey but an analysis of real-life decisions pertaining to specific graft types. We believe this is the first time such an exploratory analysis has been performed to determine which liver disease related factors influence patient decision-making surrounding extended criteria liver grafts. Future work may take the form of confirmatory analyses on additional patient cohorts.
Our data suggests that, for the most part, patients are willing to accept the expansion of the donor pool. Most patients here consented to receive most types of extended criteria grafts, although only one third consented to receive any graft type. Further analysis revealed that decompensation with ascites, the development of HCC or older age at the time of consent were repeatedly associated with the acceptance of various extended criteria graft types. The analysis revealed a trend towards the acceptance of risks of some similar (potential or definite) complications of their current disease in their transplanted graft. The presence of HCC was predictive of acceptance of grafts from those with a history of cancer. Recipient HCV infection was found to be predictive of the acceptance of an HCV positive graft in both HCV models. Recipient HBV infection was predictive of acceptance of HBV core antibody positive grafts. Interestingly from a transplant indication perspective, disease severity as measured by laboratory MELD was not found to be significant in any model.
5ConclusionsMortality risk, as measured by MELD, does not appear to influence the decisions of patients. Factors associated with poorer quality of life, namely older age, ascites, and HCC, appear to be the most important patient related factors when considering different extended criteria grafts. This analysis suggests that consent should be revisited as patients deteriorate or ameliorate on the waitlist, especially if in the form of ascites or HCC but not necessarily MELD score. Clinicians, too, should be particularly mindful of the implied desperation of such patients when considering MELD exception scores for liver transplant.