For the last decade, the combination therapy of pegylated interferon (Peg-IFN) plus ribavirin (RBV) has been considered as the standard of care treatment for chronic hepatitis C virus (HCV) infection. However, it has been associated with an increased incidence of many adverse cutaneous reactions and emergence of autoantibodies or even autoimmune diseases. We report a case of irreversible alopecia universalis (AU) with complete hair loss extended to the whole body, which started after discontinuation of Peg-IFN/RBV combination therapy for chronic HCV infection. In conclusion, this case represents an uncommon presentation of a common disease. Physicians must be aware of the potential adverse reactions of an antiviral therapy containing IFN, which might occur even after the discontinuation, and fully inform the patient at the beginning of his treatment course. We hope that interferon-free regimens will utterly supplant interferon-based therapy for most or all HCV patients avoiding the emergence of autoimmune manifestations.
Hepatitis C virus (HCV) infection is one of the main causes of chronic liver disease worldwide.1 Over the past decade, the current standard-of-care treatment has been the combination of pegylated interferon (Peg-IFN) plus ribavirin (RBV).2 With US Food and Drug Administration approval of boceprevir and telaprevir (two protease inhibitors), the standard-of-care treatment for genotype-1 infection is now Peg-IFN/RBV and a protease inhibitor.2 Pegylation is an important technique, which has greatly improved the pharmacologic profile of interferon by reducing the proteins’ sensitivity to proteolysis hence increasing their half-life.3 However, several adverse effects are associated with this treatment, among which the most common are hematological (such as anemia and thrombocytopenia), flu-like syndrome, psychiatric, endocrine, digestive and dermatological.4 The emergence of several types of autoantibodies or even autoimmune diseases has been reported during IFN-alfa treatment.5 Alopecia is a dermatologic disorder that means loss of hair. It is commonly believed to be an autoimmune disease, which might be clinically presented as a single patch of hair loss (alopecia areata; AA), complete loss of hair on the scalp (alopecia totalis) or the entire body (alopecia universalis; AU).6 We here report a case of irreversible AU after Peg-IFN α-2b and RBV treatment in a subject with chronic hepatitis C.
Case ReportA 49-year-old male patient was diagnosed as having chronic HCV infection proven by positive anti-HCV antibody and detectable HCV-RNA. He was referred to our hepatitis C outpatient clinic in January 2011. His medical history was insignificant except for arthritis of the small joints started in 2007.
When he presented to our unit in 2011, he was in good general conditions, yet, obese (BMI 31.3 kg/m2). Regarding his virological profile, he was HCV-RNA positive (55,000 IU/mL), HCV genotype 3 and serogical tests for HBV and HIV were negative. Transaminases were two times the upper limit of normal. A liver biopsy was performed showing minimal necro-inflammatory activity and fibrosis stage 2 (Ishak score). Immunological blood tests revealed a positive anti-nuclear antibody and a positive anti-thyroid peroxidase antibodies test with normal thyroid stimulating hormone value. Treatment was started with a weekly subcutaneous dose of Peg-IFNα-2b 150 μg, along with a daily oral dose of 1,200 mg of RBV. One week following the first antiviral dose, his HCV-RNA was negative (< 10 IU/mL).
No major undesired effects were reported during the treatment course, except for a mild form of pancytopenia at week 8 which required no moderation in his treatment protocol and which was resolved spontaneously by the end of treatment course that lasted for 12 weeks.
One month after the completion of the combined antiviral therapy, the patient reported a significant amount of hair loss from his scalp, which progressed to affect his eyebrows, hair on his upper and lower limbs. Hair loss continued to affect the axillary and pubic areas. By the end of the third month following treatment completion, the patient had a total loss of all body hair. The patient was evaluated by a dermatologist who diagnosed his condition as AU and prescribed a topical corticosteroid cream and heliotherapy for 6 months, without improvement.
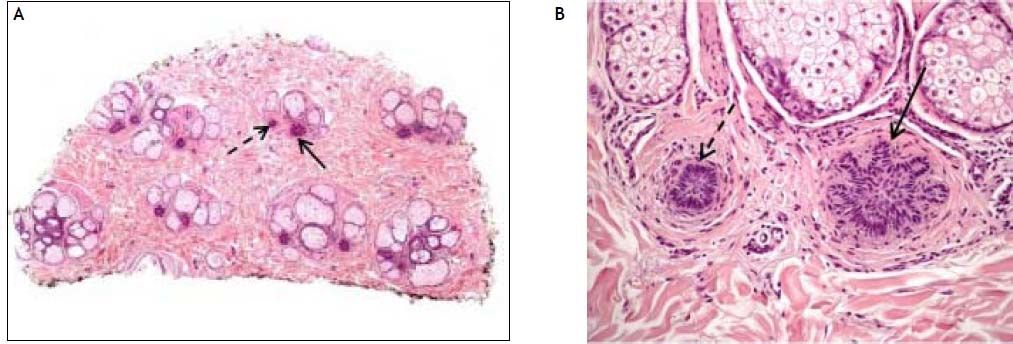
A scalp biopsy was performed 13 months after treatment discontinuation revealing absence of terminal hair follicles with increased number of miniaturized anagen hair follicles (vellus follicles), catagen and telogen germinal units (Figures 1A, 1B). Moderate perifollicular lymphocytic infiltration was also seen with slight fibroplasia. Increased number of yellow dots with few cadaverized hair (black dots) on the epidermis were seen by dermoscopy (Figure 2). The presence of yellow dots differentiates alopecia areata from trichotillomania or telogen effluvium.7 The definitive diagnosis was alopecia areata universalis in telogen arrest phase. At the time we are writing our report the patient has discontinued the antiviral therapy since 16 months, and AU still shows no improvement. The patient has tolerated this adverse effect well, and refused any systemic medications for the alopecia.
Horizontal section of scalp biopsy taken 13 months following treatment discontinuation showing absence of terminal hair follicles with increased number of miniaturized anagen hair follicles (dotted arrows), catagen and telogen germinal units (solid arrows) [H&E 4x]. B. Magnified follicle unit with miniaturized anagen hair follicle (dotted arrow) and telogen germinal unit (solid arrow) [H&E 20x].
A written informed consent was obtained from the patient to describe this report.
DiscussionAA is a disease of the anagen stage hair follicles.8 It is a clinically heterogeneous disease characterized by non-scarring hair loss on the scalp or any hairbearing surface.8 AU is the most severe variant of AA in which all scalp and body hair is lost. AA and AU are commonly believed to have an autoimmune etiology with genetic, environmental and immunological factors involved.9 Associations between AA and classical autoimmune disorders, namely, thyroid disease (including Hashimoto’s thyroiditis), myasthenia gravis, systemic lupus erythematosus and vitiligo have been previously reported.10,11 Combination Peg-IFN/ RBV therapy has been associated with an increased incidence of adverse cutaneous reactions, including injection site reactions, psoriasis, eczematoid drug reaction, sarcoidosis, mixed cryoglobulinemia, cutaneous lupus erythematosus and alopecia.12,13 Treatment with Peg-IFN has been also associated with several autoimmune disorders such as hypothyroidism, lupus-like syndrome and autoimmune hepatitis.14 Recent studies proved that Peg-IFN induces immunologic modulation (shift from Th2 immune-driven response to Th1) and stimulates the synthesis of Th1-cytokines such as IL-1, IL-2 and IFN-γ.15 Furthermore, it also increases cytotoxic T-cell activity.16 Elevated serum levels of IFN-γ and IL-2 in patients with AU have been reported and these results indicate that immune response in AU is regulated by Th1 cytokines.17,18 The pathogenetic mechanisms so far known indicate a very complex process leading to an inflammatory reaction that causes hair loss.18 It is a vicious cycle: the keratinocytes release cytokines that activate endothelial cells which, in turn, attract T-cells and macrophages that release more cytokines and so on. Autoantibodies against follicular autoantigens could be found in the serum and skin of AA patients and can occur before clinically detectable hair loss19 but there is no evidence they are pathogenic.20,21 A concrete hypothesis on the pathogenetic mechanisms of AU in our case is difficult to be formed. Presumably, in genetically predisposed individuals and under the immunemodulatory effect of Peg-IFN/RBV therapy, the hair follicle enters a cycle of autoimmune inhibition of its growth.
Although diagnosing alopecia areata might be easy, treating it is not. Our fundamental therapeutic challenge is to reduce the already established inflammatory infiltrates and to prevent further spread to unaffected hair follicles. To date, all therapeutic options do not satisfactorily meet this challenge.22 Many treatment modalities have been implicated in the treatment of AA.22 Topical corticosteroids and immunotherapy, systemic corticosteroids, systemic immunosuppresants, psoralen plus ultraviolet A photochemotherapy were among treatment options for AA. Alopecia is a physiologically distressing disorder and physicians should provide their patients with realistic advice about treatments and their effectiveness. Several forms of alopecia linked to Peg-IFN/RBV have been reported in the literature ranging from subcutaneous injection site alopecia,23 irreversible AT,24 reversible AU,25,26–28 and one case of irreversible AU.29 Interestingly, unlike all reported cases, our patient experienced hair loss one month after the discontinuation of treatment and this is quiet alarming.
To conclude, this case represents an uncommon presentation of a common disease. Physicians must be aware of the potential adverse reactions of an antiviral therapy containing IFN, which might occur even after the discontinuation, and fully inform the patient at the beginning of his treatment course. Ultimately, the advent of interferon-free regimens would represent the fulfillment of an enormous unmet need in interferon-incapable or intolerant patients. We hope that such regimens will utterly supplant interferon-based therapy for most or all HCV patients avoiding the emergence of autoimmune manifestations.
DisclosureNo conflict of interest declared.





![Horizontal section of scalp biopsy taken 13 months following treatment discontinuation showing absence of terminal hair follicles with increased number of miniaturized anagen hair follicles (dotted arrows), catagen and telogen germinal units (solid arrows) [H&E 4x]. B. Magnified follicle unit with miniaturized anagen hair follicle (dotted arrow) and telogen germinal unit (solid arrow) [H&E 20x]. Horizontal section of scalp biopsy taken 13 months following treatment discontinuation showing absence of terminal hair follicles with increased number of miniaturized anagen hair follicles (dotted arrows), catagen and telogen germinal units (solid arrows) [H&E 4x]. B. Magnified follicle unit with miniaturized anagen hair follicle (dotted arrow) and telogen germinal unit (solid arrow) [H&E 20x].](https://static.elsevier.es/multimedia/16652681/0000001300000002/v1_201906020852/S1665268119308944/v1_201906020852/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Dermoscopy [40x] showing increased number of yellow dots (dotted arrows) and few cadaverized hair (black dots) on the epidermis (solid arrows). Dermoscopy [40x] showing increased number of yellow dots (dotted arrows) and few cadaverized hair (black dots) on the epidermis (solid arrows).](https://static.elsevier.es/multimedia/16652681/0000001300000002/v1_201906020852/S1665268119308944/v1_201906020852/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





