Background. Current guidelines do not differentiate in the utilization of vasoactive drugs in patients with cirrhosis and acute variceal bleeding (AVB) depending on liver disease severity.
Material and methods. In this retrospective study, clinical outcomes in 100 patients receiving octreotide plus endoscopic therapy (ET) and 216 patients with ET alone were compared in terms of failure to control bleeding, in-hospital mortality, and transfusion requirements stratifying the results according to liver disease severity by Child-Pugh (CP) score and MELD.
Results. In patients with CP-A or those with MELD < 10 octreotide was not associated with a better outcome compared to ET alone in terms of hospital mortality (CP-A: 0.0 vs. 0.0%; MELD < 10: 0.0 vs. 2.9%, p = 1.00), failure to control bleeding (CP-A: 8.7 vs. 3.7%, p = 0.58; MELD < 10: 5.3 vs. 4.3%, p = 1.00) and need for transfusion (CP-A: 39.1 vs. 61.1%, p = 0.09; MELD < 10: 63.2 vs. 62.9%, p = 1.00). Those with severe liver dysfunction in the octreotide group showed better outcomes compared to the non-octreotide group in terms of hospital mortality (CP-B/C: 3.9 vs. 13.0%, p = 0.04; MELD > 10: 3.9 vs. 13.3%, p = 0.03) and need for transfusion (CP-B/C: 58.4 vs. 71.6%, p = 0.05; MELD > 10: 50.6 vs. 72.7%, p < 0.01). In multivariate analysis, octreotide was independently associated with in-hospital mortality (p = 0.028) and need for transfusion (p = 0.008) only in patients with severe liver dysfunction (CP-B/C or MELD > 10).
Conclusion. Patients with cirrhosis and AVB categorized as CP-A or MELD < 10 had similar clinical outcomes during hospitalization whether or not they received octreotide.
Gastrointestinal acute variceal bleeding (AVB) is a serious complication in cirrhotic patients with portal hypertension with a high related mortality.1,2 Besides hemodynamic resuscitation with crystalloids and packed red blood cell (PRBC) transfusion, current practice guidelines recommend the combination of vasoactive drugs and endoscopic therapy (ET), in addition to the use of prophylactic antibiotics as standard treatment for AVB.3–7 The vasoactive drugs, terlipressin, somatostatin, and octreotide are a standard of care for the control of AVB in patients with cirrhosis by reducing portal blood flow and portal pressure.8,9 These drugs have been associated with a lower risk of mortality as well as improved control of bleeding.10
The main determinant of AVB-related mortality is functional hepatic reserve. Child-Pugh class A (CP-A) patients have negligible mortality, whereas CP class C (CP-C) patients have a mortality rate between 20-32%.2,11 We previously reported the characteristics and outcomes of 212 patients with AVB in a 4-year period in our center. All patients received ET and antibiotic prophylaxis. Only a minority of patients (9.0%) received vasoactive drugs (oc-treotide) with no deaths reported in the subset of patients with CP-A.12
Vasoactive drugs are recommended to be started as soon as possible in suspected variceal bleeding, even before diagnostic endoscopy. However, there are no studies specifically addressing the benefits of vasoactive drugs in patients with ET depending on their baseline liver function. Furthermore, current recommendations for management of AVB do not differ for patients with different functional hepatic reserve. The use of these vasoactive drugs carries a risk of side effects and increases the cost of medical care. These considerations suggest that patients might benefit from a stratified approach to treatment, taking into account the baseline risk of the patient. Indeed, the Baveno VI workshop was entitled “Stratifying risk and individualizing care for portal hypertension”. Thus, the aims of this retrospective comparative study was to analyze the failure to control bleeding, hospital mortality and transfusion requirements among patients with AVB according to their functional hepatic reserve assessed by the CP score and the Model for End-stage Liver Disease (MELD).
Material and MethodsThis was a comparative, retrospective, observational study. The medical records of patients with liver cirrhosis presenting at our “Dr. José E. González” University Hospital with AVB were reviewed from June 2009 to July 2015. We compared two groups of patients, those receiving combined therapy (ET plus octreotide) and those receiving ET alone. The determinant for octreotide treatment was whether it was covered by the patient social insurance or, if that not was the case, if the patient had means to pay for the drug. Somatostatin and terlipressin were not used in our patients because the former is not available in our country and the latter is not covered by the patients’ social insurance.
Approval was obtained from the research and ethics committee of the School of Medicine and the “Dr. José E. González” University Hospital of the Universidad Autónoma de Nuevo León. The study was performed in compliance with the Declaration of Helsinki for biomedical research. Informed consent was obtained from all participants for the diagnostic and therapeutic maneuvers required in each case.
The reviewed medical records were included for analysis if they fulfilled the following inclusion criteria:
- •
Patient aged 18 years or older.
- •
Liver cirrhosis diagnosed by compatible clinical, laboratory, and radiologic findings or previous liver biopsy.
- •
Endoscopy performed within the 24 h of hospital admission with confirmed AVB treated with endoscopic variceal ligation (EVL) or cyanoacrylate.
- •
Prophylactic antibiotics were administered at admission or immediately after AVB was confirmed.
- •
In the group of patients receiving octreotide, this had to be administered within the first 24 h of admission.
The octreotide was administered initially at a 50 µg intravenous bolus dose and continued an intravenous infusion at 50 µg/h at least for 2 to 5 days. Patients medical files were excluded if they presented any of the following that might work as confounding factors:
- •
Non-cirrhotic portal hypertension.
- •
A history of endoscopic variceal therapy within 2 weeks before the episode.
- •
Patients with other evident source of bleeding different to esophageal or gastric varices.
- •
Insufficient or confusing clinical data from the medical records regarding the primary outcomes.
General characteristics (age, gender, etiology of cirrhosis), laboratory and clinical variables were recorded. Liver function at admission was assessed using the CP score and MELD. The CP score was determined using the classical parameters: ascites, encephalopathy, serum albumin, total bilirubin and prothrombin time. The patients were classified as CP-A (5-6 points), CP-B (7-9 points) and CP-C (> 10 points).13 The MELD score was calculated using the following formula: 3.8 log e (serum bilirubin mg/dL) + 11.2 log e (INR) + 9.6 log e (serum creatinine mg/dL) + 6.4.13
During hospital stay, the following outcomes were recorded: 5-day failure to control bleeding, in-hospital mortality, PRBC transfusion requirements, survival without rebleeding and length of hospital stay.
DefinitionsTime zero (T0) was defined as the time of hospital admission. Failure to control bleeding was defined as any occurrence of fresh hematemesis or recurrent melena (after passing normal stools) resulting in a 3 g drop in hemoglobin (9% drop of hematocrit) or need for transfusion. In-hospital mortality was defined as death from any cause during hospitalization. Survival without rebleeding was defined as the control of AVB without rebleeding or death. Need for transfusion was defined as the requirement of at least one PRBC transfusion from any cause during hospital stay. Hospital stay length was defined as the period of time in days from T0 to discharge or death.
Study aimsThe primary aim was to determine the effect of using or not octreotide in the 5-day failure to control bleeding, hospital all-cause mortality and the need for transfusion in patients with cirrhosis and AVB treated endoscopically depending on their functional hepatic reserve measured by CP score or MELD.
Secondary objectives were to determine the effect of using or not octreotide in survival without rebleeding, and hospital stay length between the octreotide and non-octreotide groups. Also, to compared the general and clinical variables between patients according to 5-day control of bleeding and hospital mortality.
Statistical analysisStatistical analyses were performed with SPSS 20.0 (IBM, Armonk, NY, USA). Baseline characteristics of patients in both groups with and without octreotide were compared with the use of Student’s t-test or the Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables, as appropriate. Data are presented as absolute value and percentages, mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate. The relationship between the different variables and the risk of developing the endpoints were analyzed by logistic regression. The contribution of each variable to the risk of developing the endpoint is reported as the odds ratio (OR) with 95% confidence interval (CI). Multivariate logistic regression models were used to calculate adjusted probabilities (aOR) of 5-day failure to control bleeding, in-hospital mortality and need for transfusion within each CP and MELD strata.
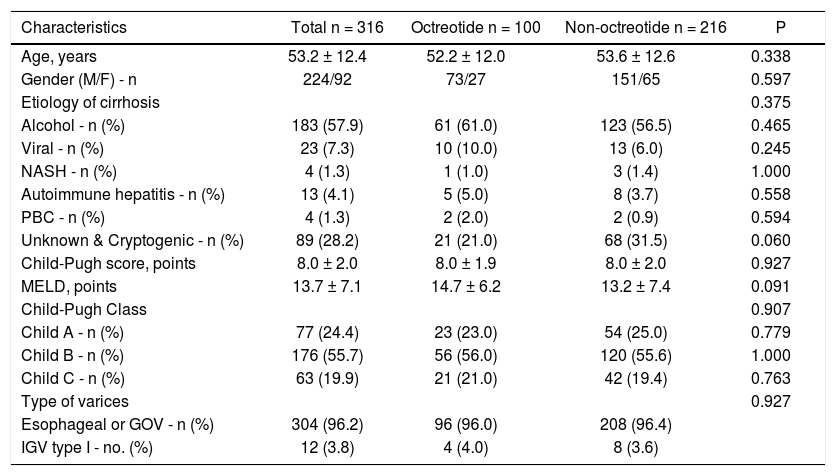
ResultsPatient characteristicsThe medical records of three-hundred and sixteen patients were retrospectively searched. Of the 316 patients, 100 were treated with ET plus octreotide (octreotide group) and 216 were treated with ET alone (non-octreotide group). All patients received prophylactic antibiotics at admission or immediately after AVB was confirmed. The study population was male dominant (70.9%) with a median age of 53.2 ± 12.4 years. Alcoholic liver disease (57.9%) was the most common cause of liver cirrhosis. Baseline characteristics of patients did not differ significantly between the two treatment groups (Table 1). There were no missing data for the variables of interest.
Baseline characteristics of patients with acute variceal bleeding according to type of treatment.
| Characteristics | Total n = 316 | Octreotide n = 100 | Non-octreotide n = 216 | P |
|---|---|---|---|---|
| Age, years | 53.2 ± 12.4 | 52.2 ± 12.0 | 53.6 ± 12.6 | 0.338 |
| Gender (M/F) - n | 224/92 | 73/27 | 151/65 | 0.597 |
| Etiology of cirrhosis | 0.375 | |||
| Alcohol - n (%) | 183 (57.9) | 61 (61.0) | 123 (56.5) | 0.465 |
| Viral - n (%) | 23 (7.3) | 10 (10.0) | 13 (6.0) | 0.245 |
| NASH - n (%) | 4 (1.3) | 1 (1.0) | 3 (1.4) | 1.000 |
| Autoimmune hepatitis - n (%) | 13 (4.1) | 5 (5.0) | 8 (3.7) | 0.558 |
| PBC - n (%) | 4 (1.3) | 2 (2.0) | 2 (0.9) | 0.594 |
| Unknown & Cryptogenic - n (%) | 89 (28.2) | 21 (21.0) | 68 (31.5) | 0.060 |
| Child-Pugh score, points | 8.0 ± 2.0 | 8.0 ± 1.9 | 8.0 ± 2.0 | 0.927 |
| MELD, points | 13.7 ± 7.1 | 14.7 ± 6.2 | 13.2 ± 7.4 | 0.091 |
| Child-Pugh Class | 0.907 | |||
| Child A - n (%) | 77 (24.4) | 23 (23.0) | 54 (25.0) | 0.779 |
| Child B - n (%) | 176 (55.7) | 56 (56.0) | 120 (55.6) | 1.000 |
| Child C - n (%) | 63 (19.9) | 21 (21.0) | 42 (19.4) | 0.763 |
| Type of varices | 0.927 | |||
| Esophageal or GOV - n (%) | 304 (96.2) | 96 (96.0) | 208 (96.4) | |
| IGV type I - no. (%) | 12 (3.8) | 4 (4.0) | 8 (3.6) |
Data are shown as absolute value (%) and means ± standard deviation. Both treatment groups received endoscopic therapy. Virus category includes patients with viral hepatitis alone and patients with viral hepatitis and alcohol NASH: Non-alcoholic steatohepatitis. PBC: Primary biliary cholangitis. MELD: Model for End-stage Liver Disease. GOV: Gastroesophageal varices. IGV: Isolated Gastric Varices.
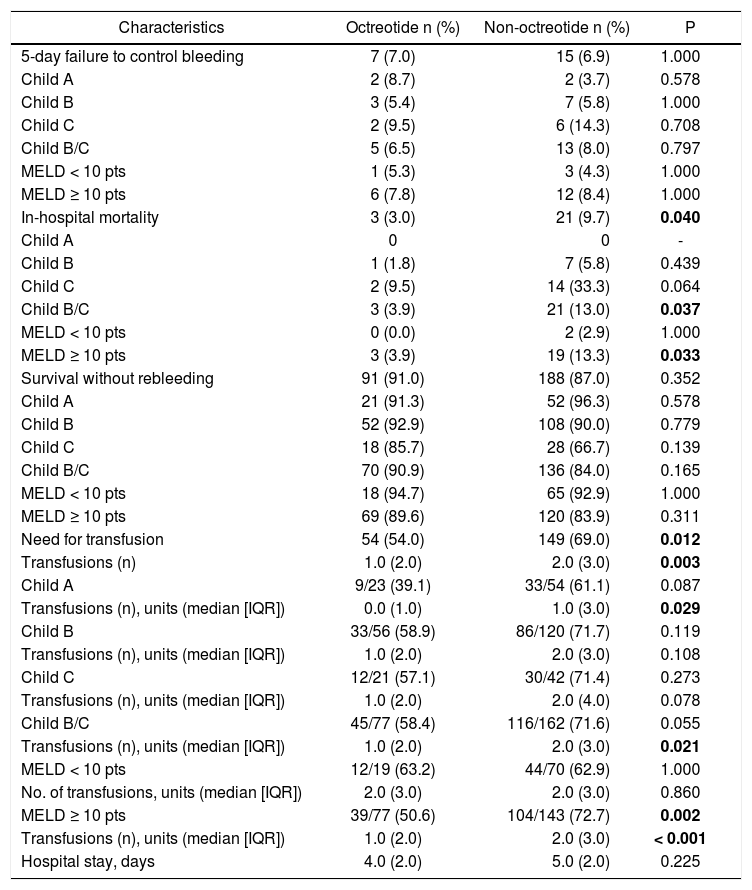
The 5-day failure to control bleeding between groups was 7.0% in the octreotide group compared to 6.9% in the non-octreotide group, p = 1.000. No differences were observed in any strata defined by CP or MELD (Table 2).
Outcomes in patients with and without octreotide, in the overall series and in different subgroups.
| Characteristics | Octreotide n (%) | Non-octreotide n (%) | P |
|---|---|---|---|
| 5-day failure to control bleeding | 7 (7.0) | 15 (6.9) | 1.000 |
| Child A | 2 (8.7) | 2 (3.7) | 0.578 |
| Child B | 3 (5.4) | 7 (5.8) | 1.000 |
| Child C | 2 (9.5) | 6 (14.3) | 0.708 |
| Child B/C | 5 (6.5) | 13 (8.0) | 0.797 |
| MELD < 10 pts | 1 (5.3) | 3 (4.3) | 1.000 |
| MELD ≥ 10 pts | 6 (7.8) | 12 (8.4) | 1.000 |
| In-hospital mortality | 3 (3.0) | 21 (9.7) | 0.040 |
| Child A | 0 | 0 | - |
| Child B | 1 (1.8) | 7 (5.8) | 0.439 |
| Child C | 2 (9.5) | 14 (33.3) | 0.064 |
| Child B/C | 3 (3.9) | 21 (13.0) | 0.037 |
| MELD < 10 pts | 0 (0.0) | 2 (2.9) | 1.000 |
| MELD ≥ 10 pts | 3 (3.9) | 19 (13.3) | 0.033 |
| Survival without rebleeding | 91 (91.0) | 188 (87.0) | 0.352 |
| Child A | 21 (91.3) | 52 (96.3) | 0.578 |
| Child B | 52 (92.9) | 108 (90.0) | 0.779 |
| Child C | 18 (85.7) | 28 (66.7) | 0.139 |
| Child B/C | 70 (90.9) | 136 (84.0) | 0.165 |
| MELD < 10 pts | 18 (94.7) | 65 (92.9) | 1.000 |
| MELD ≥ 10 pts | 69 (89.6) | 120 (83.9) | 0.311 |
| Need for transfusion | 54 (54.0) | 149 (69.0) | 0.012 |
| Transfusions (n) | 1.0 (2.0) | 2.0 (3.0) | 0.003 |
| Child A | 9/23 (39.1) | 33/54 (61.1) | 0.087 |
| Transfusions (n), units (median [IQR]) | 0.0 (1.0) | 1.0 (3.0) | 0.029 |
| Child B | 33/56 (58.9) | 86/120 (71.7) | 0.119 |
| Transfusions (n), units (median [IQR]) | 1.0 (2.0) | 2.0 (3.0) | 0.108 |
| Child C | 12/21 (57.1) | 30/42 (71.4) | 0.273 |
| Transfusions (n), units (median [IQR]) | 1.0 (2.0) | 2.0 (4.0) | 0.078 |
| Child B/C | 45/77 (58.4) | 116/162 (71.6) | 0.055 |
| Transfusions (n), units (median [IQR]) | 1.0 (2.0) | 2.0 (3.0) | 0.021 |
| MELD < 10 pts | 12/19 (63.2) | 44/70 (62.9) | 1.000 |
| No. of transfusions, units (median [IQR]) | 2.0 (3.0) | 2.0 (3.0) | 0.860 |
| MELD ≥ 10 pts | 39/77 (50.6) | 104/143 (72.7) | 0.002 |
| Transfusions (n), units (median [IQR]) | 1.0 (2.0) | 2.0 (3.0) | < 0.001 |
| Hospital stay, days | 4.0 (2.0) | 5.0 (2.0) | 0.225 |
Bold numbers represent statistical significance. MELD: Model for End-stage Liver Disease. IQR: Interquartile range.
Overall, patients in the octreotide group had a lower hospital mortality compared with those not receiving octreotide, 3.0% vs. 9.7% (p = 0.04) (Table 2). Mortality in CP-A patients and in patients with a MELD below 10 was negligible and not impacted by octreotide treatment (CPA: 0.0 vs. 0.0%; and MELD < 10: 0.0 vs. 2.9%, p = 1.00). Those with severe liver dysfunction in the octreotide group presented lower hospital mortality than those in the non-octreotide group (Table 2).
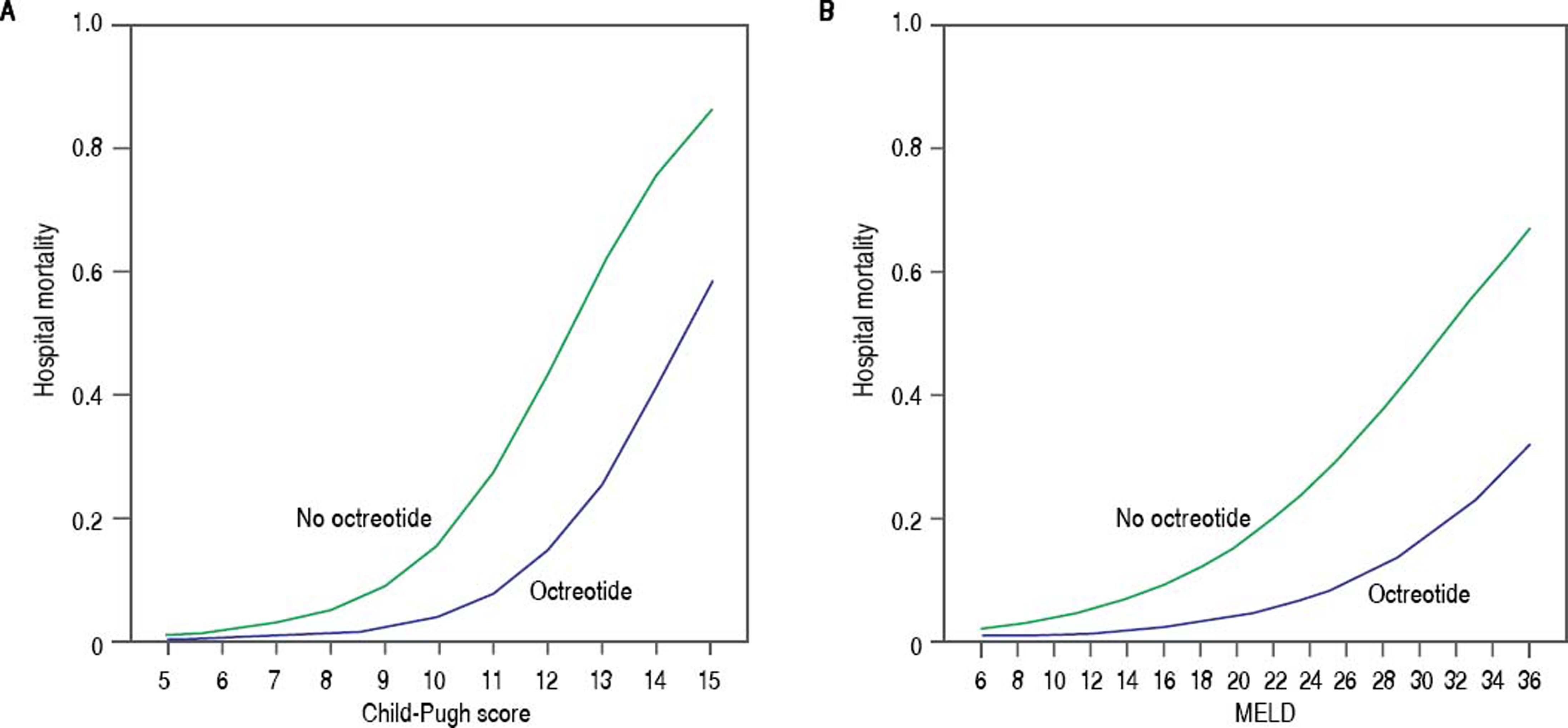
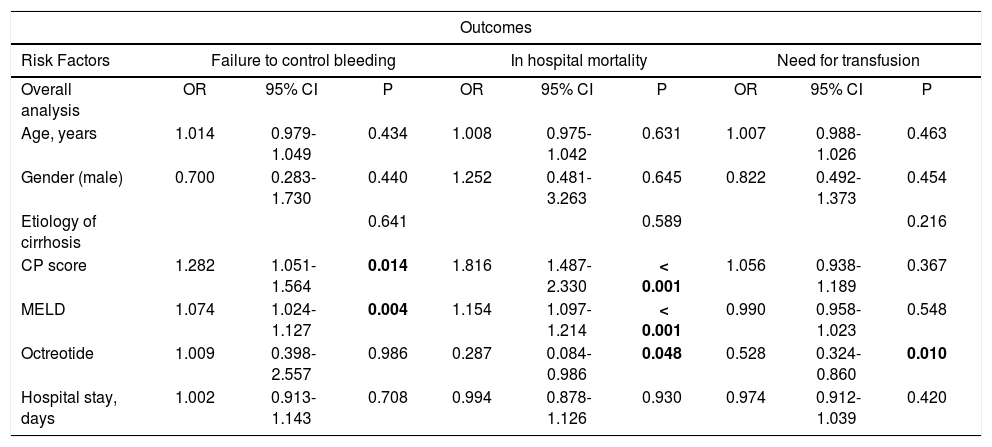
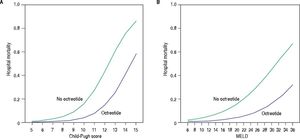
Univariate analysis showed that octreotide was associated with a decreased risk of mortality (Table 3, OR 0.287, 95% CI 0.084-0.986; p = 0.048). This association between octreotide treatment and survival remained significant at multivariate analysis (Table 4: aOR 0.179, 95% CI 0.044-0.735; p = 0.017). The predicted hospital mortality in octreotide and non-octreotide groups across the spectrum of CP score and MELD showed that the benefit of using octreotide was observed in patients with CP-B/C and in patients with MELD > 10 (Figure 1). Subanalysis by CP and MELD strata showed in multivariate analysis that only in those with severe liver disease (CP-B/C or MELD ≥ 10) octreotide use was associated with an improved prognosis (Table 4).
Univariate analysis for primary clinical outcomes in cirrhotic patients with acute variceal bleeding.
| Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Factors | Failure to control bleeding | In hospital mortality | Need for transfusion | ||||||
| Overall analysis | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Age, years | 1.014 | 0.979-1.049 | 0.434 | 1.008 | 0.975-1.042 | 0.631 | 1.007 | 0.988-1.026 | 0.463 |
| Gender (male) | 0.700 | 0.283-1.730 | 0.440 | 1.252 | 0.481-3.263 | 0.645 | 0.822 | 0.492-1.373 | 0.454 |
| Etiology of cirrhosis | 0.641 | 0.589 | 0.216 | ||||||
| CP score | 1.282 | 1.051-1.564 | 0.014 | 1.816 | 1.487-2.330 | < 0.001 | 1.056 | 0.938-1.189 | 0.367 |
| MELD | 1.074 | 1.024-1.127 | 0.004 | 1.154 | 1.097-1.214 | < 0.001 | 0.990 | 0.958-1.023 | 0.548 |
| Octreotide | 1.009 | 0.398-2.557 | 0.986 | 0.287 | 0.084-0.986 | 0.048 | 0.528 | 0.324-0.860 | 0.010 |
| Hospital stay, days | 1.002 | 0.913-1.143 | 0.708 | 0.994 | 0.878-1.126 | 0.930 | 0.974 | 0.912-1.039 | 0.420 |
Bold numbers represent statistical significance. CP score and MELD: The risk for the occurrence of clinical outcomes increase per point. CP: Child-Pugh. MELD: Model for End-stage Liver Disease. OR: Odds Ratio. CI: Confidence interval.
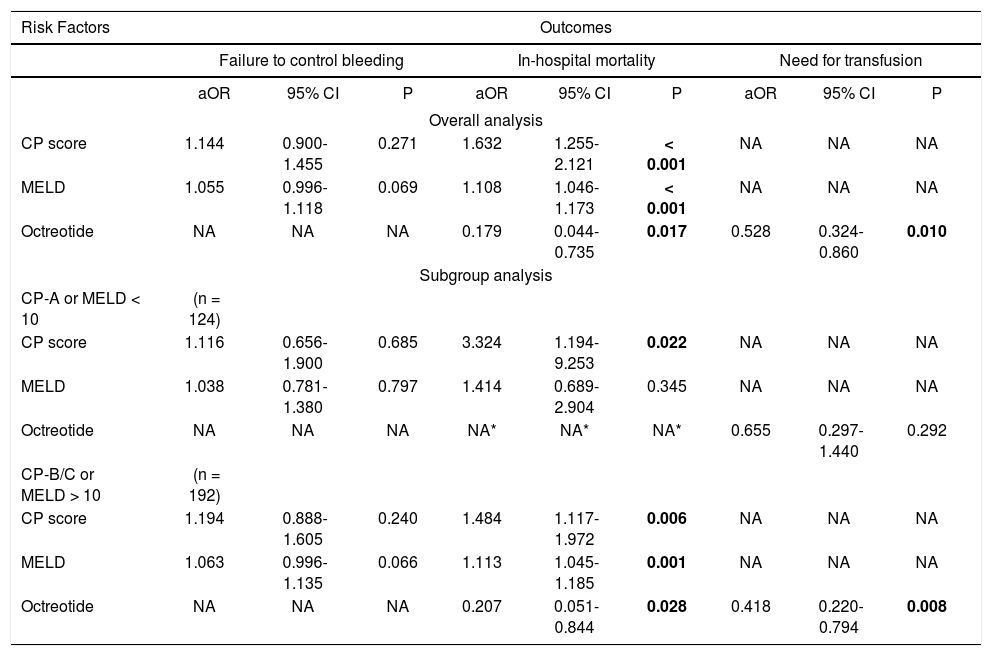
Multivariate analysis for primary clinical outcomes in cirrhotic patients with acute variceal bleeding.
| Risk Factors | Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure to control bleeding | In-hospital mortality | Need for transfusion | |||||||
| aOR | 95% CI | P | aOR | 95% CI | P | aOR | 95% CI | P | |
| Overall analysis | |||||||||
| CP score | 1.144 | 0.900-1.455 | 0.271 | 1.632 | 1.255-2.121 | < 0.001 | NA | NA | NA |
| MELD | 1.055 | 0.996-1.118 | 0.069 | 1.108 | 1.046-1.173 | < 0.001 | NA | NA | NA |
| Octreotide | NA | NA | NA | 0.179 | 0.044-0.735 | 0.017 | 0.528 | 0.324-0.860 | 0.010 |
| Subgroup analysis | |||||||||
| CP-A or MELD < 10 | (n = 124) | ||||||||
| CP score | 1.116 | 0.656-1.900 | 0.685 | 3.324 | 1.194-9.253 | 0.022 | NA | NA | NA |
| MELD | 1.038 | 0.781-1.380 | 0.797 | 1.414 | 0.689-2.904 | 0.345 | NA | NA | NA |
| Octreotide | NA | NA | NA | NA* | NA* | NA* | 0.655 | 0.297-1.440 | 0.292 |
| CP-B/C or MELD > 10 | (n = 192) | ||||||||
| CP score | 1.194 | 0.888-1.605 | 0.240 | 1.484 | 1.117-1.972 | 0.006 | NA | NA | NA |
| MELD | 1.063 | 0.996-1.135 | 0.066 | 1.113 | 1.045-1.185 | 0.001 | NA | NA | NA |
| Octreotide | NA | NA | NA | 0.207 | 0.051-0.844 | 0.028 | 0.418 | 0.220-0.794 | 0.008 |
Bold numbers represent statistical significance. CP score and MELD: The risk for the occurrence of clinical outcomes increase per point. CP: Child-Pugh. MELD: Model for End-stage Liver Disease. aOR: adjusted Odds Ratio. CI: Confidence interval. NA: Not applicable. * There were no recorded deaths in the octreotide group in patients classified as CP-A or MELD < 10, so it was not possible to calculate the adjusted risk by multivariate logistic regression.
The proportion of patients needing transfusion was 54.0% in the octreotide group compared to 69.0% in the non-octreotide group (p = 0.012). The median number (IQR) of PRBC units transfused was 1.0 (2.0) in the octreotide group and 2.0 (3.0) in the non-octreotide group (p = 0.003). In univariate analysis, octreotide was the only factor independently associated with the need for transfusion (Table 3: OR 0.528, 95% CI 0.324-0.860; p = 0.010).
Patients with CP-A as well as those with MELD < 10 in the non-octreotide group presented no significant difference compared with those in the octreotide group in the need for transfusion (Table 2). When we compared the number of transfusions between groups based in the baseline hepatic function by CP class and MELD, there was some significant difference (Table 2). However, octreotide was not independently associated with the need for transfusion of 2 or more PRBC units in the subgroup of patients with CP-A (OR 0.272, 95% CI 0.056-1.312; p = 0.105) and MELD below 10 (OR 1.192, 95% CI 0.414-3.431; p = 0.745). Moreover, among those with severe liver disease, octreotide was a protective factor independently associated with the need for transfusion of 2 or more PRBC units (CP-B/C: OR 0.496, 95% CI 0.262-0.940; p = 0.032; and MELD ≥ 10: OR 0.270, 95% CI 0.124-0.588; p = 0.001). In addition as shown in table 4, the need for transfusion was only modified by octreotide in the subgroup of patients with severe liver disease (CPB/C or MELD ≥ 10).
Secondary analysisNo difference in survival without rebleeding and inhospital stay length between the octreotide and non-octreotide groups either in the overall analysis or by stratifying patients according to the CP class or MELD was found (p > 0.05). We also compared the general and clinical variables between patients according to 5-day control of bleeding, and in-hospital mortality (Table 5). At univariate analysis, both CP score and MELD predicted failure to control bleeding and mortality (Table 3); in multivariate analysis CP score and MELD were independently associated only to the mortality outcome (Table 4).
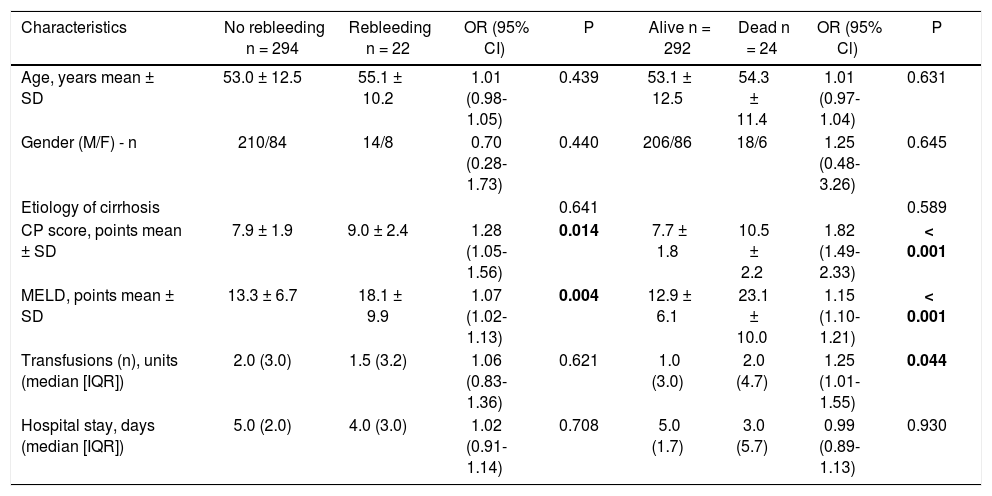
Comparison of general and clinical variables between patients according to control of bleeding and hospital mortality.
| Characteristics | No rebleeding n = 294 | Rebleeding n = 22 | OR (95% CI) | P | Alive n = 292 | Dead n = 24 | OR (95% Cl) | P |
|---|---|---|---|---|---|---|---|---|
| Age, years mean ± SD | 53.0 ± 12.5 | 55.1 ± 10.2 | 1.01 (0.98-1.05) | 0.439 | 53.1 ± 12.5 | 54.3 ± 11.4 | 1.01 (0.97-1.04) | 0.631 |
| Gender (M/F) - n | 210/84 | 14/8 | 0.70 (0.28-1.73) | 0.440 | 206/86 | 18/6 | 1.25 (0.48-3.26) | 0.645 |
| Etiology of cirrhosis | 0.641 | 0.589 | ||||||
| CP score, points mean ± SD | 7.9 ± 1.9 | 9.0 ± 2.4 | 1.28 (1.05-1.56) | 0.014 | 7.7 ± 1.8 | 10.5 ± 2.2 | 1.82 (1.49-2.33) | < 0.001 |
| MELD, points mean ± SD | 13.3 ± 6.7 | 18.1 ± 9.9 | 1.07 (1.02-1.13) | 0.004 | 12.9 ± 6.1 | 23.1 ± 10.0 | 1.15 (1.10-1.21) | < 0.001 |
| Transfusions (n), units (median [IQR]) | 2.0 (3.0) | 1.5 (3.2) | 1.06 (0.83-1.36) | 0.621 | 1.0 (3.0) | 2.0 (4.7) | 1.25 (1.01-1.55) | 0.044 |
| Hospital stay, days (median [IQR]) | 5.0 (2.0) | 4.0 (3.0) | 1.02 (0.91-1.14) | 0.708 | 5.0 (1.7) | 3.0 (5.7) | 0.99 (0.89-1.13) | 0.930 |
Bold numbers represent statistical significance. Data are shown as absolute value and means ± SD or median (IQR) as indicated. CP: Child-Pugh. MELD: Model for End-stage Liver Disease. OR: Odds ratio. CI: Confidence interval. SD: Standard deviation. IQR: Interquartile range.
The combination of vasoactive agents with EVL is standard therapy for AVB,9,10,14-18 but it is still uncertain if all patients need vasoactive treatment or if this could be avoided in low-risk patients. This is especially relevant in environments in which access to drug therapy is limited by its cost. In this study, we have shown that the probability of failure to control bleeding, mortality and need for transfusion during hospitalization in CP-A or MELD < 10 patients was very low regardless of whether they received or not octreotide. Patients with severe liver dysfunction (CP-B/C or MELD > 10) in the octreotide group showed better outcomes compared to patients not receiving octreotide in terms of mortality and PRBC transfusion requirements.
The current recommendations of using combined therapy of vasoactive drugs and ET in the setting of AVB, lies on the evidence provided by studies that assessed the 5-day success rate.14,15,18,19 However, most of these studies used endoscopic sclerotherapy which is inferior to EVL;20 in addition, they did not stratify outcomes based on the grade of liver disease severity.
Several studies have shown that combination therapy improves control of bleeding compared with ET alone, though the effect in survival is still controversial.9,10,14–18 In addition there is little information on whether patients with preserved liver function (CP-A or MELD < 10), who are at very low risk of treatment failure benefit from combination therapy. In agreement with our results, Berreta, et al. reported 0% mortality in both treatment groups (combined therapy vs. ET alone) in the subset of patients with CP-A.21 They showed that the benefit of using combined therapy was the reduction of initial hemostatic failure and rebleeding in the CP-C group and those presenting with active bleeding. In a previous study, our group also reported 0% mortality in patients with CP-A even though only a very small proportion of patients in that study received octreotide.12
A previous randomized placebo-controlled doubleblind study comparing endoscopic variceal sclerotherapy alone and in combination with octreotide in patients with low-risk cirrhosis (excluding patients with refractory as-cites, CP-C, and chronic encephalopathy) and AVB reported a significant difference in the rate of 5-day rebleeding, a shorter hospital stay and number of PRBC units transfused in the combined treatment group.22 However, even though this study focused on a group of low-risk cirrhotic patients, it did not differentiate between very low-risk CP-A and CP-B patients, and the study did not provide data stratifying by MELD. In addition, due to the small number of patients and since just one patient from each group died during the study, they were not able to provide conclusions regarding mortality. Another randomized placebo-controlled double-blind study reported a significantly higher rate of “survival without rebleeding” at five days in all three CP classes with combined therapy as compared with monotherapy alone; however, mortality was unaffected;17 octreotide was also associated with an overall reduction in transfusion requirements; however, these results were not analyzed stratifying patients according to CP class and MELD.17 In a trial comparing sclero-therapy plus somatostatin versus sclerotherapy alone, Avgerinos, et al. reported an improvement in the overall 5-day failure of therapy (defined as any of the following: transfusion of an excess of blood products; hematemesis; hemodynamic instability; use of rescue therapy; or death) with combination therapy in overall analysis (35 vs. 55%, p = 0.004), as well as in the subset of patients with a CP-A (2 patients vs. 9 patients, p = 0.021).18 However, they do not report specific mortality data in the CP-A subgroup; in addition, the number of CP-A patients was small. Despite the retrospective nature of our study, we collected 124 patients with very low risk (CP-A or MELD < 10) which greatly exceeds the number of low-risk patients as compared to previous reports.12,18,22
Because mortality in CP-A patients with AVB has been reported to be 0%,2,11,21 which is in accordance with our results, we speculate that this very low risk subgroup of patients (CP-A or MELD < 10) may have a better hemodynamic adaptation response to an episode of AVB with no additional benefit from the splanchnic vasoconstrictor properties of vasoactive drugs. These variations in the physiopathological mechanisms with respect to vascular hemodynamics have already been proven to vary depending on the severity of liver disease.23–27
One surprising finding in our study was that octreotide had no impact on control of bleeding but still showed a positive effect in terms of mortality and transfusion requirements. Since most of our patients were endoscopically treated with EVL (> 95%), the most likely explanation is that EVL is a highly effective method for hemostasis, at least within the first five days of admission. This is supported by the data from a meta-analysis demonstrating that EVL is superior in control bleeding compared to sclerotherapy.20 In addition, in accordance with our results, a recent randomized controlled trial comparing EVL plus somatostatin infusion versus EVL plus placebo did not find any advantage in control of AVB at 5 days.28 However, confounders we could not reliably capture (i.e. the incidence of spontaneous bacterial peritonitis or septic complications) could be a posible explanation for the difference in mortality in this retrospective review.
Due to the inconclusive evidence related to the cost-effectiveness of vasoactive drugs in AVB in patients categorized as “very low risk”, studies with a six-week follow-up comparing the cost-effectiveness of combined therapy (ET + terlipressin, somatostatin or octreotide) with ET alone are crucial before to give a recommendation in this subgroup of patients. However, the strengths of this study lies in the fact that we provide thorough data on CP and MELD stratified patients and the benefits of using octreotide in AVB in a large number of patients treated with EVL. The only determinant of octreotide treatment was the socioeconomic status of the patients, which determined whether they could pay or not for the drug. Thus, the clinical baseline characteristics were comparable between groups treated or non-treated with octreotide.
A limitation of our study is the potential indication bias introduced by octreotide treatment. Though the strongest prognostic determinant in AVB is the baseline liver function,29,30 the determinants for the use of octreotide might have introduced unknown bias that could not be accounted for in the multivariate analysis. Also, though we adjusted the effects of octreotide by the most relevant predictors in a multivariate analysis, there might be residual confounding not captured by the standard variables that were collected in our study (e.g. cardiovascular comorbidities, history of prior variceal bleedings, portal vein thrombosis, hepatocarcinoma, etc).
In summary, this study presents the benefits of octreotide in patients with cirrhosis presenting with AVB according to liver disease severity by CP score and MELD. Patients with cirrhosis and preserved liver function (CP-A or MELD < 10) presenting with AVB may not benefit from octreotide during hospitalization in terms of failure to control bleeding, mortality and the need for transfusion.
AcknowledgmentsWe thank Sergio Lozano-Rodríguez, M.D. for his help in editing the manuscript.
Conflicts of InterestThe authors declare that they have no conflict of interest or financial disclosures.
FundingWe received no funding in the preparation of this manuscript.
Abbreviations- •
aOR: adjusted odds ratio.
- •
AVB: Acute variceal bleeding.
- •
CI: Confidence interval.
- •
CP: Child-Pugh.
- •
CP-A: Child-Pugh class A.
- •
CP-B: Child-Pugh class B.
- •
CP-C: Child-Pugh class C.
- •
ET: Endoscopic therapy.
- •
EVL: Endoscopic variceal ligation.
- •
IQR: Interquartile range.
- •
MELD: Model for End-stage Liver Disease.
- •
OR: Odds ratio.
- •
PRBC: Packed red blood cell.
- •
SD: Standard deviation.