The circadian oscillations of many physiological processes provide an endogenous temporal program for the adaptive synchronization of mammals to the fluctuating external world. The lack of exposure to light causes the circadian system to undergo a process of dark adaptation similar to dark adaptation in the visual system. The aim of the present work was investigate the effect of acute treatment of constant darkness on mitochondrial ATP synthase activities and membrane fluidity in liver from male rat. We found that ATP synthase activity was not changed by the treatment. However ATPase activity and membrane fluidity were significantly diminished and pH gradient driven by ATP hydrolysis was incremented, in comparison from samples from rats kept on normal light/dark cycles. Additionally, the treatment of constant darkness diminishes the passive proton permeability of the inner mitochondrial membrane. In conclusion constant darkness induces a more efficient coupling between proton transport and catalysis, and increment the efficiency of the enzyme because the ratio of ATP synthase/ATPase activity was higher. These results exhibited the physiological adaptation of liver mitochondria to acute treatment of constant darkness in order to satisfy the cellular energy demand.
The most reliable and strongest environmental 24 h-synchronizer of self-sustaining oscillations or circadian biological rhythms in mammals is the light-dark cycle, provided by the sun and stational natural season of the planet. Circadian rhythms provide an endogenous temporal program for the adaptive synchronization of physiology and behavior to the fluctuating external world. In mammals, photic information from the retina is transmitted to the suprachiasmatic nuclei (SCN) of the hypothalamus via direct and indirect neural pathways.1 The SCN clock then synchronizes overt rhythms in physiology and behavior, probably through both synaptic connections and humoral signals. For instance the SCN controls the rhythmic synthesis of melatonin in the pineal organ,2 which is involved in the transduction of photoperiodic information, and appears to modulate a multiplicity of neural and endocrine functions. It is known that periods of constant darkness cause stimulation of melatonin secretion from the pineal gland.3 Other tissues that have synchronization-like effects, like the brain, liver, but the SCN appears to have the master circadian pacemaker.4,5
Neuroendocrine responses to environmental stimuli, such as light or its absence, can influence metabolic and immune responses through the pineal gland. For instance, it has been reported that constant darkness decreases the blood glucose level in rats6 and causes hypertrophy and increased cellularity of the thymus.7
On the other hand, metabolism and components of the internal clock that drives circadian rhythms are intimately related. For example, up to % 1 of the transcriptome is under circadian regulation,5,8-10 and mitochondrial respiratory efficiency undergoes daily rhythmic oscillations.11 Particularly, in the liver the onset of the expression of genes involved in energy metabolism precedes the activity onset to prepare for energy storage. Similarly, the expression of glucose transporters and rate-limiting enzymes in hexose catabolism starts to rise before, and peaks precisely at, the time when most feeding occurs.9
Mitochondria provides cellular energy via oxidative phosphorylation, using the multisubunit complexes of the respiratory chain to create a transmembrane potential, which drives ATP synthesis by the F1F0-ATP synthase. That enzyme is also capable of working as an ATPase, hydrolyzing ATP to pump protons from the mitochondrial matrix to the intermembrane space.
Considering that constant darkness treatment influences metabolism the aim of the present work was investigate the changes in enzymatic activities of ATP synthase and mitochondrial membrane properties in liver from rats kept under constant darkness for a period of 72 hours.
MethodsExperiments in animals were approved by the laboratory animal care committee of our institution, and were conducted according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985).
40 Adult male Wistar rats weighting 200-220 g were maintained in rooms with automatic temperature (22 ± 1 °C) and received standard laboratory chow (Purina) and water ad libitum. They were randomly separated into two groups of 20 animals each: control and experimental. The control group was housed under a 12 h light and 12 h dark cycle. The darkness period started at 20:00 h. Experimental group was maintained in continuous darkness for 72 h. Also we monitored the quantity of food intake.
After treatment, animal were sacrificed by decapitation at 8:00 h and liver was immediately dissected for mitochondria isolation. Liver was homogenized with a teflon-on-glass homogenizer Potter-Elvehjem in 35 ml cold SHE buffer (250 mM sucrose, 10 mM Tris (pH 7.4), 1 mM ethylene glycol bis(β-aminoethyl ether)-N,N,N’N’-tetraacetic acid (EGTA)) and kept on ice. The homogenate was centrifuged at 600 xg for 5 min at 4°C. The pellet was discarded, and the supernatant was centrifuged at 8,000 xg for 10 min at 4°C. The foamy layer at the top of the supernatant was removed. The mitochondrial pellet was washed with SHE buffer containing 0.1% fatty acid-free serum albumin and finally resuspended in SHE to contain about 40 mg/mL protein. Outer membrane mitochondria were removed according to Greenawalt12 and tightly coupled rat liver submitochondrial particles were obtained as previously described.13
ATP synthesis determinationATP synthesis was assayed with a coupled enzymatic reaction at 37 °C. The reaction mixture (0.5 mL) contained 125 mM KCl, 25 mM sucrose, 40 mM Hepes (pH 7.5), 5 mM MgCl2, 4 mM ADP, 5 mM inorganic phosphate, 0.1 mM EGTA, 1 mM NADH, 20 mM glucose, 46 units of hexokinase.14 This mixture was preincubated at 37 °C for 2 min in Erlenmeyer flasks under constant shaking, followed by the addition of submitochondrial particles (1 mg of protein). The reaction was arrested with 50 μL of stop mix (25 mM EDTA, 2 μM carbonyl cyanide 3-chlorophenylhydrazone (CCCP)), and 25 μg oligomycin). Afterwards, the reaction mixture was boiled for 10 min and centrifuged at 5;000 xg for 5 min. 0.5 mM NADP and 30 units of glucose-6-phosphate dehydrogenase were added to the supernatant and incubated for 10 min at 30 °C. The absorbance of the samples was recorded at 340 nm.
ATPase activity determinationATP hydrolysis was assayed by the release of inorganic phosphate. The standard reaction medium (1 mL) contained 125 mM KCl, 40 mM Hepes/KOH (pH 8.0), 0.1 mM EGTA, 3 mM ATP, 5 mM MgCl2. The reaction was initiated by the addition of submitochondrial particles (0.25 mg of protein).15 It was quenched with 200 mL of cold 30% (w/v) trichloroacetic acid and inorganic phosphate was measured by the colorimetric method described by Sumner.16
pH gradientThe pH gradient driven by ATP hydrolysis were determined by the fluorescence quenching of 1 μM ACMA (9-amino-6-chloro-2-methoxyacridin). Briefly, submitochondrial particles (2 mg of protein) were incubated at 37 °C in a medium (2 mL) containing 125 mM KCl, 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS) (pH 7.5), 5 mM MgCl2, 0.1 mM EGTA, 1 μM ACMA, 3 mM ADP and 5 mM inorganic phosphate. After stabilization of the signal, membrane was energized with 3 mM ATP or succinate. ACMA fluorescent quenching is directly related to pH gradient, i.e., the higher values of fluorescent quenching, the higher values of pH gradient.17
Membrane fluidity determinationsMembrane fluidity was estimated from the excimer to monomer fluorescence intensity ratio (Ie/Im) of the fluorescent probe 1,3 dipyrenylpropane (DPP) incorporated in submitochondrial particles. Briefly, 0.25 mg of mitochondrial protein and 0.1 nmol DPP were mixed with 10 mM Tris–HCl buffer (pH 7.8). The mixtures were incubated in darkness at 4 °C for 5 hours, in order to achieve maximal incorporation of the fluorescent probe to the membranes. Fluorescence was measured at 24 °C on a Perkin Elmer fluorescence spectrometer, LS50B. The fluorophore was excited at 329 nm and the monomer and excimer fluorescence intensities were read at 379 and 480 nm, respectively. From these readings, the excimer to monomer fluorescence intensity ratio (Ie/Im) was calculated. Fluorescence corrections obtained from readings of membranes without DPP were applied to all fluorescence values.18
Passive Proton Permeability determinationFor passive proton permeability assays the procedure described by Groen, et al.19 was utilized. Briefly, mitochondrial incubations (1 mg of protein) for oxygen consumption by means of a Clark-type electrode were performed at 30 °C, under constant stirring. The reaction medium (1 mL) contained 125 mM KCl, 20 mM MOPS (pH 7.5), 2 mM MgCl2, 2 mM inorganic phosphate, 3.5 μg rotenone, 1 μg oligomycin, 40 μM N-ethylmaleimide. The reaction was initiated by the addition of 3 mM succinate as the substrate. Respiration rate was stimulated by the addition of increasing amounts of carbonyil cyanide 4-trifuoromethoxyphenylhydrazone (FCCP) concentrations.
Other assaysCitrate synthase activity was assayed in a reaction medium (1 mL) consisting of 100 mM Tris/HCl (pH 8.1), 40 μg/mL 5,5’-dithio-bis(2-nitrobenzoic acid), 1 mM oxaloacetate, 0.3 mM acetyl coenzyme A and 0.4 % of triton X-100. After 3 min of incubation, the reaction was initiated by adding 1 mg of mitochondrial proteins and the change in optical density at 412 nm was recorded for 3 min.
Mitochondrial membrane protein was measured by the method of Lowry20 in the presence of 0.066% sodium deoxycholate using bovine serum albumin as standard.
Statistical analysisThe results were expressed as mean ± S.E.M. The statistical differences were evaluated by oneway analysis of variance followed by the least significant difference test.
ResultsAlthough much is known about effects of light on the circadian system, little is known about constant darkness on metabolism and mitochondrial enzymatic activities. In order to assess the biochemical implications of the acute constant darkness treatment, we measured rates of coupled ATP synthesis. In mitochondria the ATP catalytic sites are internal and it is therefore advantageous to prepare inverted vesicles from these membranes for assays of ATP synthase activities.
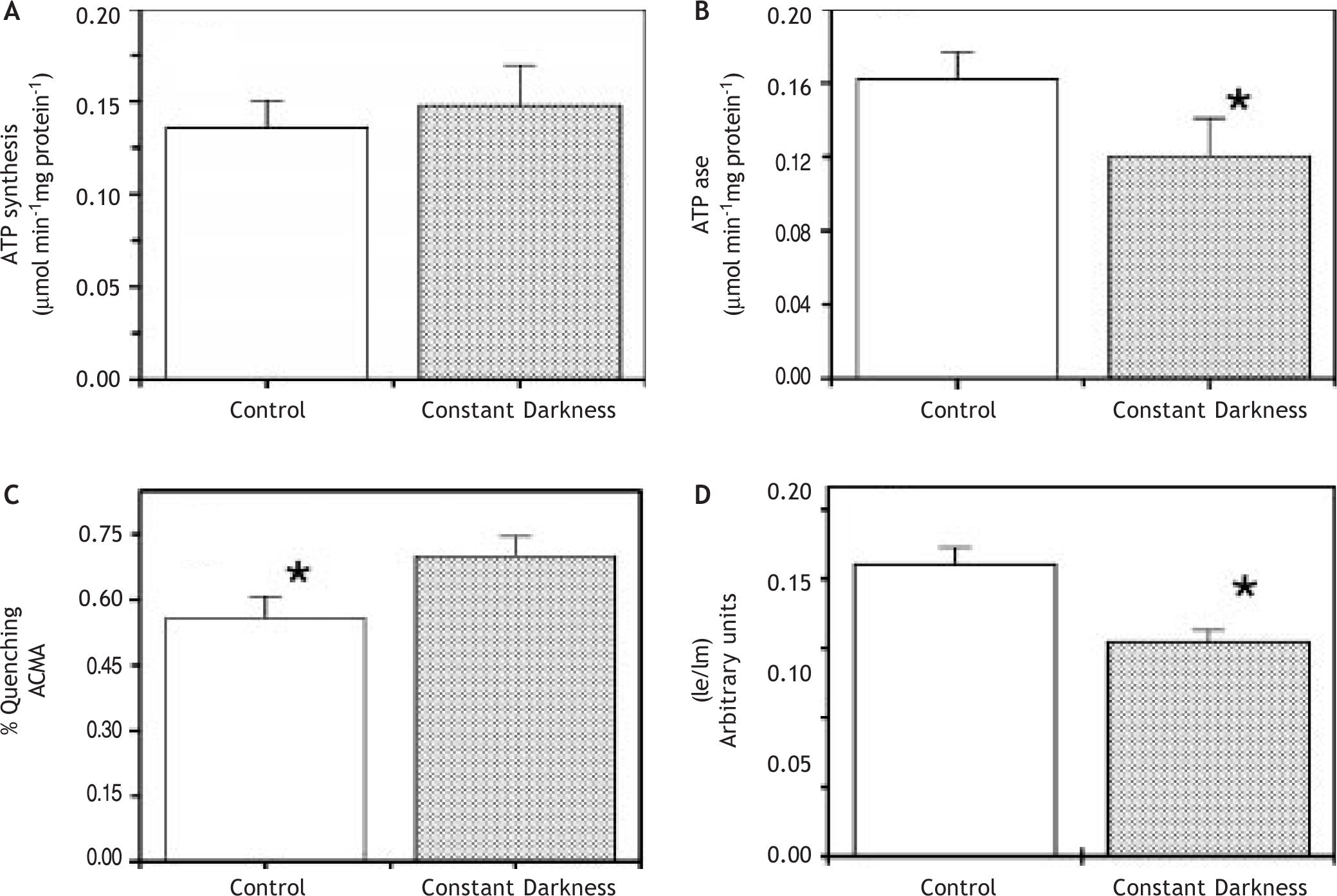
Figure 1A exhibited that ATP synthase activity in submitochondrial particles from liver was not altered by acute treatment of constant darkness, with respect to controls. Since the F1F0 ATP synthase is a reversible ATP synthase/ATPase we also made determinations of ATPase activity and pH gradient driven by ATPase activity. ATPase activity was significantly diminished by constant darkness acute treatment (Figure 1B).
ATP synthase activity (A), ATPase activity (B), % ACMA fluorescence quenching (C) and excimer/monomer intensity fluorescence ratio (D) in mitochondria (A) and submitochondrial particles (B, C, D) from rats kept under constant darkness and normal cycles of light/dark. Enzymatic activities and membrane fluidity determinations were done as described in Methods. The data shown are mean ± S.E. *p < 0.05.
pH gradient driven by ATP hydrolysis was monitored with the fluorescent dye ACMA, where quenching of fluorescence indicates the generation of a proton gradient across the membrane.17
pH gradient driven by ATP hydrolysis has been extensively used as an indication of the enzyme proton channel function and of coupling between transport and catalysis.21,22Figure 1C showed that acute treatment of constant darkness induced a higher pH gradient in mitochondrial membranes, in spite of a diminution of ATPase activity. Similar results were obtained with succinate as oxidizable substrate (not shown).
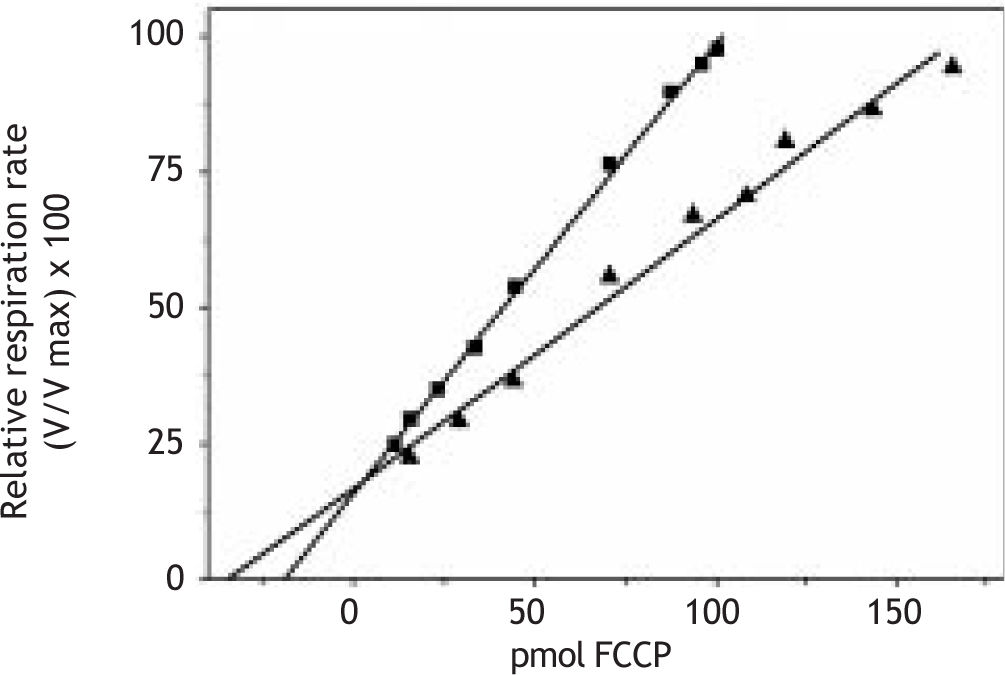
These unexpected and apparently contradictory results prompt us to study the mitochondrial membrane properties. Thus, we made determinations of membrane fluidity with the fluorescent probe dipyrenylpropane. Figure 1D shows lower excimer to monomer (Ie/Im) dipyrenylpropane ratio in samples from constant darkness than in control, indicating a decrease in membrane fluidity. To explore further the membrane properties we measured passive proton permeability through titration of respiration with FCCP. In these experiments extrapolation to zero respiration gives an amount that is directly related to the passive proton permeability of the membranes. Under our experimental conditions, the relative rate of oxygen consumption (V/Vmax x 100) is a linear function of the FCCP amount, up to a ratio V/Vmax = 1, for each type of mitochondria tested. As shown in Figure 2 the passive proton leak through inner mitochondrial membranes is equivalent to 19 and 35 pmol FCCP for, respectively, dark-treated and control rats. This data clearly showed that the acute treatment induce a low permeability of the inner mitochondrial membrane.
In contrast, the activity of citrate synthase, a commonly used matrix mitochondrial marker enzyme, was not modified by the darkness treatment (it was 0.225 ± 0.03 nmol min-1 mg of protein-1 and 0.245 ± 0.04 nmol min-1 mg of protein-1 for dark-treated and control rats, respectively.
DiscussionThe data presented in this paper show that the changes in the mitochondrial ATP synthase activities, membrane fluidity and the proton gradient driven by ATP hydrolysis in livers from rats subjected to a 72 h period of darkness is related to low passive proton permeability of the inner mitochondrial membrane. On the other hand, the diminution in membrane fluidity detected in samples of dark-treated rats does not alter the functioning of ATP synthase activity. Our results show that constant darkness acute treatment made more efficient the coupling between proton transport and catalysis. Additionally this treatment induces a higher ratio of ATP synthase/ATPase activities (1.6) in comparison with controls (1.13). Therefore, under an acute treatment of constant darkness there is a low hydrolytic state and a high synthetic state of the enzyme. Other factors, not explored in this work, could contribute to the efficiency of ATP synthase, such as the ATPase inhibitor protein. The darkness treatment does not modify the citrate synthase activity, therefore, it appears that this treatment specifically affects the ATP synthase.
The acute treatment of constant darkness slightly diminished the food consumption in rats, since the daily ingestion was 22.5 ± 0.6 g. and the one of the rats under a 12/12 light-dark cycle was 24 ± 0.7 g. Similar results have been found in male Wistar albino rats subjected to constant darkness.23 In mice liver, the decrease in the consumption of food induced by constant darkness is accompanied by an increase in the expression of genes related to fat catabolism, particularly the murine procolipase (mClps) and pancreatic lipase-related protein 2. Additionally blood glucose was decreased significantly by constant darkness.6,24 Together these studies demonstrate that the induction of mClps expression and pancreatic lipase-related protein by constant darkness accomplishes both satiety reduction and the activation of fat catabolism.
Although it has been established that light is a dominant synchronizing cue for circadian system, the system is fully functional without light input due to circadian clocks running endogenously. In the rat, the length of the period is longer that 24 h. Under constant darkness, the circadian clocks freerun expressing their endogenous periodicity referred to as the “free-running period”. Therefore the release of rats into 72 h constant darkness preserves normal rhythmicity of the circadian system but the phase of the rhythms is phase-delayed, which help organisms to optimize their metabolism in a fluctuating environment. Therefore, rats under this situation could fit better if fat catabolism is induced and mitochondrial ATP synthase works mainly as ATP synthase.
AcknowledgmentsEMR and ALRI were supported by a postgraduate fellowship from CONACYT-México.