Edited by: Sonia Roman
Last update: January 2023
More infoThe purpose of this study was to evaluate the effect of abdominal obesity and chronic inflammation on risk of non-alcoholic fatty liver disease (NAFLD) among Chinese population.
Materials and MethodsOverall, 50776 staff from the Kailuan Group who participated in and finished physical examinations between 2006 and 2007 were included in the cohort study. Their medical information was collected and they were followed after examination. The correlations of waist-to-height ratio (WHtR) or serum high-sensitivity C-reactive protein (hs-crp) with NAFLD were analyzed. Then, we categorized all participants into four groups: non-abdominal obesity and non-chronic inflammation group, abdominal obesity and non-chronic inflammation group, non-abdominal obesity and chronic inflammation group, abdominal obesity and chronic inflammation group, and non-abdominal obesity and non-chronic inflammation group was used as a control group. The combined effects of abdominal obesity and chronic inflammation with NAFLD were analyzed using the Cox proportional hazard regression model.
ResultsAfter a mean follow-up of 5.59±1.79 years, a total of 15451 NAFLD cases occurred. We found the WHtR and hs-crp increase the risk for NAFLD, respectively. Compared with the non-abdominal obesity and non-chronic inflammation group, the risk of NAFLD was significantly increased in the abdominal obesity and non-chronic inflammation group (HR 1.21, 95%CI 1.11-1.32), non-abdominal obesity and chronic inflammation group (HR 1.32, 95%CI 1.27-1.38), abdominal obesity and chronic inflammation group (HR 1.60, 95% CI 1.52-1.70). And, a significant interaction effect was found of abdominal obesity and chronic inflammation on NAFLD.
ConclusionsIn this study, it was demonstrated in the Chinese population that both abdominal obesity and chronic inflammation increase the risk of NAFLD, and there is an interaction between the two factors in the incidence of NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders characterized by hepatic fat accumulation, inflammation, and hepatocyte injury, which will result in cirrhosis and even hepatocellular carcinoma in the long-term. In the past two decades, the prevalence rate of NAFLD in China has been increasing dramatically, from 23.8% to 32.9%, much higher than that in developed countries [1]. Due to the popularization of HBV vaccination programs in China and the emergence of various metabolic diseases induced by western lifestyles, the types of liver disease have changed a great deal. NAFLD, gradually, has replaced viral hepatitis as the most common chronic liver disease in China [2].
Previous studies found obesity and chronic inflammation are associated with NAFLD. Epidemiological studies showed that the risk of NAFLD in patients with obesity is significantly higher than that in the general population. A meta-analysis made by Pang et al. further confirmed that the incidence of NAFLD is closely associated with abdominal obesity, and the association may be independent of body mass index (BMI). Waist-height ratio (WHtR) is a specific measure of abdominal obesity and is related to risk of NAFLD [3–5]. Other research suggested that chronic inflammation also plays an important role in the occurrence and development of NAFLD. Kuppan et al. conducted a cross-sectional study and demonstrated that high level of high-sensitivity C-reactive protein (hs-crp), which is a prototype inflammatory marker, is responsible for the increased risk of NAFLD [6-7]. Obese individuals had increased levels of hs-crp and elevated white blood cell counts, which may increase the risk of NAFLD and its complications [8]. However, previous studies only focus on the separate effect of abdominal obesity or chronic inflammation on NAFLD, and the co-effect of the two factors has not been fully demonstrated.
Therefore, in the present study, we investigated to determine the impact of WHtR and hs-crp on the risk of developing NAFLD by using a large community-based prospective study cohort from Kailuan Study.
2Methods2.1Study design and populationThe Kailuan Study is an ongoing prospective community-based cohort study conducted in Tangshan, China. All participants in the Kailuan Study are employees and retirees of the Kailuan Group. Details of the study design and procedure have been described elsewhere [9]. At baseline, 101,510 participants (81,110 males and 20,400 females; aged 18-98 years) were recruited, underwent clinical and laboratory examinations, and completed a questionnaire interview (June 2006 to October 2007) at 11 hospitals affiliated with the Kailuan Group. Subsequent examinations involving anthropometric, cardiovascular risk factor measures, and self-reported questionnaires (including income, educational level, drinking and so on) occurred approximately biennially.
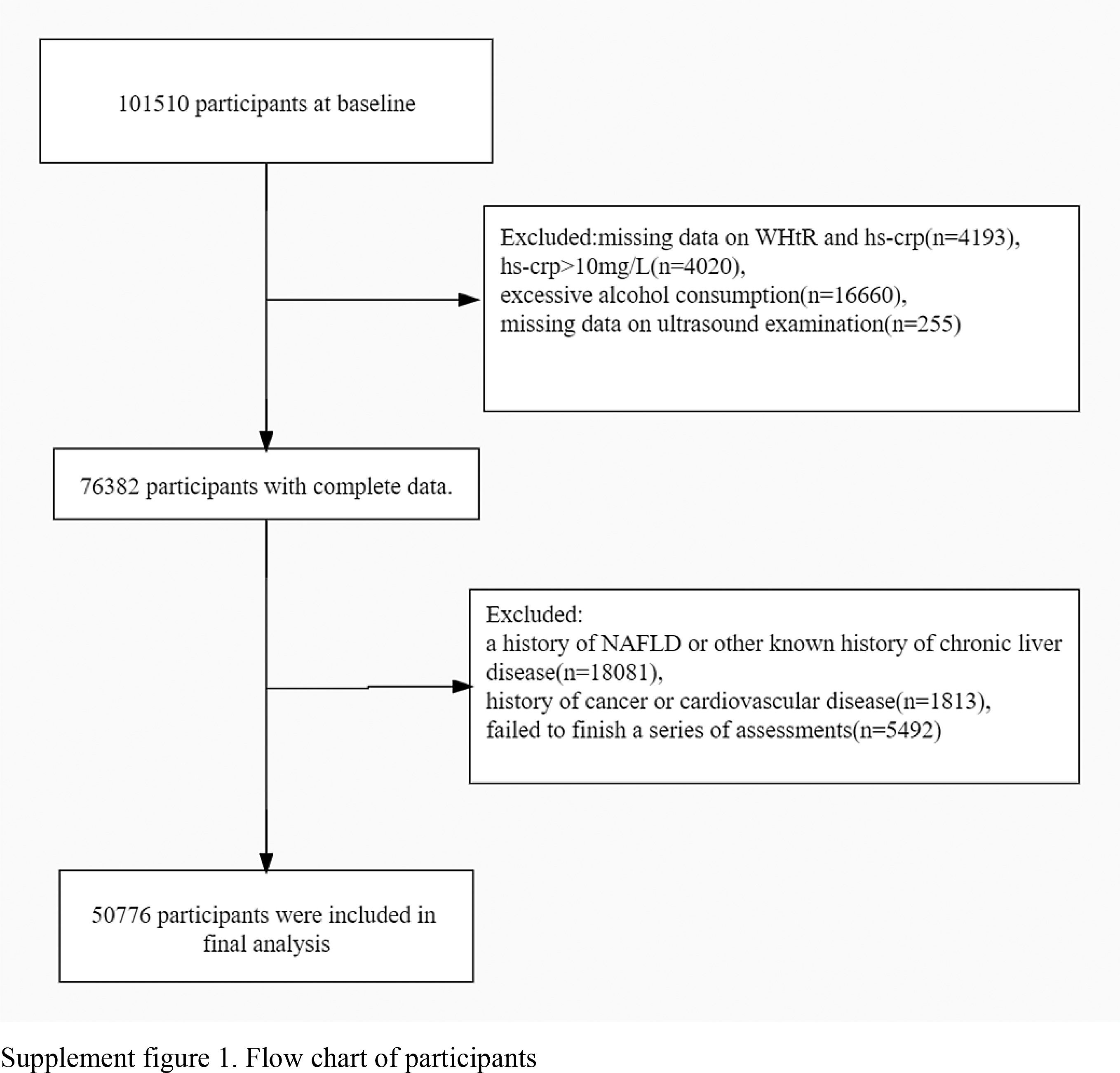
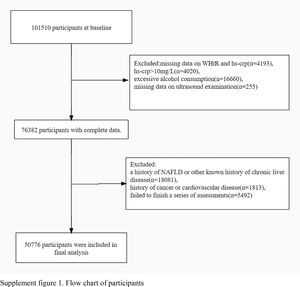
We excluded participants without baseline information on WHtR or hs-crp (n=4193), and under the following conditions including hs-crp>10 mg/L (n=4020), alcohol consumption more than 70g/week for females or 140g/week for males (n=16660), and missing data on ultrasound examination (n=255). Additionally, participants were excluded if a history of NAFLD or other known history of chronic liver disease such as autoimmune hepatitis or viral hepatitis (HBsAg positive or anti-HCV antibody positive, etc.) or those using hepatotoxic drugs (n=18081), a history of cancer and cardiovascular disease (n=1813) or failed to finish a series of assessments(n=5492). Finally, a total of 50776 individuals were enrolled in the present study (Figure 1).
The study was performed according to the guidelines of the Helsinki Declaration and was approved by the Ethics Committee of Kailuan General Hospital (approval number: 2006-05). All participants were agreed to take part in the study and provided informed written consent.
2.2Data collectionDuring the visits, anthropometric measurements including height, weight, waist circumference (WC), and blood pressure (BP) were recorded according to a standard protocol. Height and WC were averaged to 0.1 cm and weight was averaged to 0.1 kg. BMI is calculated as weight (kg) divided by height squared (m2). WHtR is calculated by dividing each WC by the participant's height. In accordance with World Health Organization classification, abdominal obesity was defined as WHtR ≥0.5 and non-abdominal obesity was defined as WHtR <0.5 [10].
Blood samples from antecubital vein were obtained in the morning after a night of fasting and then transfused into vacutainers containing ethylene diamine tetraacetic acid (EDTA). Samples were centrifuged at 3,000 g for 10 min at room temperature. After separation, the blood samples were immediately frozen at -80 °C for storage for further laboratory examinations. The level of hs-crp was measured using a commercial, high-sensitivity nephelometry assay (Cias Latex CRP-H, Kanto Chemical Co. Inc), with a detection limit of 0.1 mg/L. In-house intra- and inter-assay CVs for hs-crp were 6.53% and 4.78%, respectively. All the blood variables were measured using an autoanalyzer (Hitachi 747; Hitachi) at the central laboratory of the Kailuan hospital (Tangshan, China). According to baseline hs-crp levels and American Heart Association cardiovascular disease risk cutpoints: hs-crp <1 mg/L, low; hs-crp 1–3 mg/L, moderate; and hs-crp ≥3 mg/L, elevated [11]. And hs-crp ≥3 mg/L was considered as chronic inflammation.
Due to the limitation of research conditions at that time, serum insulin level was not directly collected and HOMA-IR could not be evaluated. Insulin sensitivity was evaluated using the triglyceride-glucose index (TyG index). And the TyG index was calculated as ln (fasting triglyceride [mg/dL] × fasting blood glucose [mg/dL]/2).
2.3Assessment of outcomesFollow-up ended at the first record of NAFLD event, all-cause death, at the last date of follow up or at the end of follow-up on 31 December 2013, whichever came first. Diagnosis of NAFLD was based on the presence of at least two of the following three abnormal findings after excluding the individuals with excess alcohol intake(more than 70 g/week for females or 140g/week for males) and autoimmune hepatitis or viral hepatitis according to the National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology: (1) diffusely increased echogenicity of the liver relative to the kidney; (2) ultrasound beam attenuation; (3) poor visualization of intrahepatic structures [12]. Abdominal ultrasonography was performed by using a high-resolution B-mode topographical ultrasound system with a 3.5 MHz probe (PHILIPS HD-15, US).
2.4Other covariatesParticipants were classified as “current” or “never” according to whether they smoked at least one cigarette weekly for over one year. In terms of physical activity, participants were divided based on whether they took activity more than three times a week with each time lasting more than 30 min or not. Education level and general information of the participants were collected from questionnaires as baseline in 2006. Diabetes mellitus was defined based on a fasting blood glucose value ≥7.0 mmol/L or the participant had previous medical treatment for diabetes or a history of diabetes. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or the participant had previous administration of antihypertensive medications or a history of hypertension.
2.5Statistical analysisContinuous variables were compared using analysis of variance or the Kruskal-Wallis test according to distribution, and categorical variables were compared with the chi-square test. A correlation between WHtR and hs-crp was analyzed by Pearson's chi-squared test.
To investigate the relationship of abdominal obesity and chronic inflammation with NAFLD, the participants were stratified into groups based on WHtR (<0.5 and >0.5) and hs-crp levels (<1 mg/L, 1-3 mg/L, ≥3 mg/L). In the analysis of the combined effect, all subjects were assigned into four groups: non-abdominal obesity and non-chronic inflammation (G1), abdominal obesity and non-chronic inflammation (G2), non-abdominal obesity and chronic inflammation (G3), abdominal obesity and chronic inflammation (G4). G1 was used as a control group. The incidence of NAFLD was calculated. Hazard ratios (HR) and 95% confidence intervals (CI) of WHtR, hs-crp alone, and their combination on NAFLD were analyzed by the Cox proportional hazard model. And the Cox models and found that the proportional hazards assumption was satisfied. Finally, exposure factors were introduced into the multivariate model as an interaction term to test the interaction between abdominal obesity and chronic inflammation [13]. We calculated the relative excess risk due to interaction (RERI), proportion of disease attributable to interaction (AP) and synergy index for interaction (SI) [9,14-15]. Interaction was considered significant when the 95%CI of RERI, AP and SI did not comprise 0.0 and 1, separately. In the Cox and interaction models, the adjusted variables included age, gender, BMI, TyG index, smoke (never/current), physical activity (yes/no), hypertension (yes/no), diabetes mellitus (yes/no), high school or above (yes/no). Subgroup analyses were performed based on age, sex, BMI and diabetes mellitus. All analyses were done with SAS (version 9.4), at a two-tailed alpha level of 0.05.
3Results3.1General characteristics of the study subjectsAmong the 50776 subjects included in the statistical analysis, there were 38169 men and 12607 women, with a mean age of 49.40±12.57 years, a mean WHtR of 0.51±0.06, and a median hs-crp of 0.69 (0.25-1.72) mg/L. The baseline characteristics of the participants stratified by different abdominal obesity status and chronic inflammation status were shown in Table 1. The correlation coefficient between WHtR and hs-crp was r=0.175.
Baseline characteristics of the population
Note: BMI: body-mass index, WC: waist circumference, WHtR: waist to height ratio; FBG: fasting blood glucose, TyG index: triglyceride-glucose index, hs-crp: high sensitivity C-reactive protein. G1 non-abdominal obesity and non-chronic inflammation, G2 abdominal obesity and non-chronic inflammation, G3 non-abdominal obesity and chronic inflammation, G4 abdominal obesity and chronic inflammation.
The mean follow-up period was 5.59±1.79 years involving 15451 cases (including 11088 men and 4363 women). The overall incidence of NAFLD was 54.46 per 1000 person-years (for male and for female). The G4 group had highest incidence of NAFLD (table 2).
HR and 95% CI for risk of NAFLD by WHtR and hs-crp categories.
Note: * The model was adjusted for age, sex, BMI, TyG index, smoke, hypertension, diabetes mellitus, physical activity, education. +Additional adjustment for WHtR/hs-crp. G1 non-abdominal obesity and non-chronic inflammation, G2 abdominal obesity and non-chronic inflammation, G3 non-abdominal obesity and chronic inflammation, G4 abdominal obesity and chronic inflammation.
As shown in Table 2, the results of multivariate Cox proportional hazard analysis indicated the risk of NAFLD. Compared with those with hs-crp<1 mg/L group, risk of NAFLD was significantly higher in those with hs-crp 1-3 mg/L group (HR 1.14, 95% CI 1.10-1.18) and hs-crp ≥3 mg/L group (HR 1.26, 95% CI 1.21-1.32). After correction for the same confounding factors, the risk of NAFLD in the WhtR>0.5 group (HR 1.31, 95% CI 1.26-1.37) was higher, compared with those with WhtR≤0.5 group.
The participants were classified into four groups based on the levels of WHtR and hs-crp. The result of the multivariate Cox proportional hazard analysis indicated that after the adjustments of confounding factors, the HR in the G4 group (HR 1.60, 95% CI 1.52-1.70) was increased compared with that in the G1 group. In addition, the HR in the G4 group was higher than that in the G2 (HR 1.21, 95%CI 1.11-1.32) group and the G3 (HR 1.32, 95%CI 1.27-1.38) group (Table 2).
3.3Subgroup analysis on risk of NAFLDRegarding previous study on factors associated with NAFLD, age, sex, obesity which is usually presented as BMI index and diabetes mellitus were considered as potential factors. We therefore performed four subgroup analyses to observe the effect of factors on development of NAFLD. As shown in Table 3, the result of the multivariate Cox proportional hazard model indicated that the risk in the G4 group in the male and female was increased 1.45 times (95% CI 1.36-1.55) and 1.94 times (95% CI 1.75-2.16) compared with the G1 group, respectively. The results of the remaining three subgroup analyses are similar.
The combined effect of WHtR and hs-crp on NAFLD.
Note: * The model was adjusted for age, sex, BMI, TyG index, smoke, hypertension, diabetes mellitus, physical activity, education. G1 non-abdominal obesity and non-chronic inflammation, G2 abdominal obesity and non-chronic inflammation, G3 non-abdominal obesity and chronic inflammation, G4 abdominal obesity and chronic inflammation.
After the adjustment conducted for other factors, the following three measured values of additive interaction were all significant: RERI 0.28, 95% CI 0.19-0.37; AP 0.17, 95% CI 0.12-0.22; SI 1.86, 95% CI 1.44-2.39. In addition, the multiplicative interaction was statistically significant (P <0.05). All indicated a significant synergistic interaction between WHtR and hs-crp on the risk of NAFLD.
4DiscussionIn this large prospective cohort study, we found that abdominal obesity and chronic inflammation were separately significantly associated with a high risk of NAFLD after adjustment was made for potential confounders. Furthermore, we found that the synergistic interaction between abdominal obesity and chronic inflammation could make considerable effects in the development of NAFLD.
Our results indicated that abdominal obesity was an independent risk factor of NAFLD. In our study, participants with abdominal obesity had a higher risk of NAFLD than those without abdominal obesity (HR 1.31, 95%CI 1.26-1.37), which was independent from the influence of BMI. In previous studies, the most commonly used indicator of general obesity was BMI. The skeleton of Chinese population is relatively small, but the abdominal fat is relatively thick. So, WHtR may be a more suitable index to evaluate the degree of obesity in Chinese population. WHtR retains the basic characteristic of waist circumference and is less affected by height. Results of various studies also showed that the diagnostic efficiency of WHtR for NAFLD is better than that of BMI, WC, waist hip rate (WHR) and other anthropometric indexes [16–17]. A meta-analysis of 21 cross-sectional or cohort studies found that although both abdominal obesity and general obesity increase the risk of NAFLD, the effect of abdominal obesity on NAFLD is significantly greater than that of BMI [5]. A cross-sectional study involving 4,872 subjects from northern Iran reported that WHtR is more effective in the diagnosis of NAFLD than WHR [4]. The above results suggest that the risk of NAFLD in the individuals with abdominal obesity may be higher than that in the individuals with general obesity. Therefore, abdominal obesity must be taken into consideration when studies specialized in relation between adiposity and NAFLD.
Chronic inflammation is reported to be associated with the development of NAFLD. In the present study, we found that hs-crp was an independent risk factor for NAFLD. Compared with the hs-crp<1 mg/L group, the HR of NAFLD increased to 1.14 (95%CI 1.10-1.18) and 1.26 (95%CI 1.21-1.32) when hs-crp was in 1-3 mg/L and higher than 3 mg/L, respectively, showing that the HR of NAFLD progressively increased with the rise of hs-crp level (P for trend <0.05). Many studies have reported that hs-crp is closely related to the occurrence and development of NAFLD, but most of these are cross-sectional studies [6-7,18]. A Korean study, including more than 4,000 male subjects with an average follow-up period of 7 years, demonstrated that even if hs-crp is at a normal high level, the risk of NAFLD increases with the high levels of hs-crp, which further confirms our conclusion that chronic inflammation is a risk factor for NAFLD [19]. NAFLD is considered to be a manifestation of metabolic syndrome in the liver, and its pathogenesis is closely related to oxidative stress and associated with chronic inflammatory activity. And hs-crp has been regarded as a factor that can reflect chronic inflammation. Patients with reduced antioxidant capacity had higher levels of hs-crp. Patients with NAFLD are also reported to have low antioxidant capacity [20].
In this study, we found that abdominal obesity and chronic inflammation had combined effect on NAFLD, and that the interaction effect between abdominal obesity and chronic inflammation on NAFLD was significant. The HR of NAFLD caused by the two factors simultaneously (hs-crp ≥3 mg/L and WHtR >0.5) was increased to 1.60 (95%CI 1.52-1.70) compared with that upon non-abdominal obesity and non-chronic inflammation (hs-crp <3 mg/L and WHtR ≤0.5), and much higher than that of a single factor (abdominal obesity and non-chronic inflammation group HR 1.21, 95%CI 1.11-1.32; non-abdominal obesity and chronic inflammation HR 1.32, 95%CI 1.27-1.38). Similar results were obtained in the subgroup analyses. To the best of our knowledge, this is the first study to report a combination of abdominal obesity and chronic inflammation leading to an increasing risk of NAFLD [21–23]. Obesity and elevated serum hs-crp synergistically increase insulin resistance, and obesity may affect insulin resistance through both inflammation dependent and independent pathways [24]. Insulin resistance is the central to the development of NAFLD [25]. In addition, we also found that there was an interaction between chronic inflammation and abdominal obesity, suggesting a positive synergistic effect. This will remind us that in screening for early NAFLD, besides focusing on individuals with metabolic syndrome and abdominal obesity and chronic inflammation also play roles in NAFLD risk.
The possible pathogenesis for the close association between abdominal obesity combined with chronic inflammation and NAFLD is complex and has not been fully understood, which may be attributed to insulin resistance and oxidative stress. On the one hand, abdominal obesity can easily lead to liver steatosis that can be achieved by increasing free fatty acids to the liver and changing insulin levels. During obesity, macrophages infiltrate in adipose tissues and secrete a large number of inflammatory factors, such as tumor necrosis factors, leptin, adiponectin and so on, resulting in adipose tissue inflammation. Inflammation can inhibit the insulin signaling pathway of adipocytes, induce insulin resistance and promote the accumulation of lipids in the liver [26–28]. On the other hand, the oxidative stress response increased by the accumulation of triglycerides in the liver can act independently on the liver and drive inflammatory cytokines to cause liver damage, resulting in a decrease in antioxidant capacity and an increase in hs-crp level [29–31]. Abdominal obesity can induce insulin resistance by increasing inflammation and then lead to the pathogenesis of NAFLD, while chronic inflammation can also directly damage the liver. The two factors interact with each other, leading to the occurrence of disease.
Despite the large sample size and the community-based nature of this study, several limitations should be noted. First, NAFLD was assessed by ultrasonography with lower accuracy than liver biopsy, yet ultrasonography is regarded as a safe, accurate, and convenient tool to evaluate the presence of NAFLD in epidemiological surveys. Second, due to the limited research conditions, other indicators reflecting obesity cannot be measured, such as thickness of abdominal subcutaneous fat, visceral fat area and so on. Third, as insulin was not measured throughout the survey period, insulin resistance, such as that evaluated by Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), could not be examined. Finally, this study only used single measurement data, and whether the dynamic changes of anthropometric and laboratory data would affect the incidence of NAFLD still need to be further discussed.
ConclusionOur study corroborated that chronic inflammation and abdominal obesity can increase the risk of NAFLD in Chinese population, and there is an interaction between the two factors on the incidence of NAFLD. Our results support the view that there is an independent as well as a synergistic effect on NAFLD between body fat accumulation and chronic inflammation.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding sourcesNone.
Authors’ contributionsDongna Zhao and Haozhe Cui wrote the main manuscript text and conceived and designed the study. Zhiqing Shao analyzed the data and carried out a literature search. Liying Cao performed the manuscript review. All authors have read and approved the content of the manuscript.
The authors thank all the members of the Kailuan Study Team for their contributions and the participants who contributed their data.