Spur cell anemia (SCA) is an acquired form of non-autoimmune hemolytic anemia that occurs in advanced liver disease. It is characterized by the presence of acanthocytes or spur cells, spiculated erythrocytes whose shortened life span causes anemia that is unresponsive to transfusion. SCA has been regarded as a rare condition with an ominous prognosis for which the only known cure is liver transplantation, but recent prospective studies have demonstrated the existence of a milder form of SCA in which there are smaller numbers of acanthocytes, but which is nevertheless associated with hemolysis and poor outcomes. This form of SCA appears to be considerably more common than the severe classical variant. The conventional understanding of the pathogenesis of SCA is that abnormalities of lipid metabolism are the primary event driving the formation of spur cells. However, the studies that underpin this theory are based on small numbers of patients with heterogeneous clinical features and inconsistent use of nomenclature for dysmorphic red blood cells. In this review, we discuss the evolution of the current understanding of SCA and therapeutic strategies that have been employed based on this understanding. Our goal is to raise awareness of this understudied condition that has significant implications for patient outcomes. Furthermore, we highlight the need for rigorous, contemporary research into the underlying cause or causes of SCA in order to develop an effective therapy for this disorder.
A number of morphologic abnormalities of erythrocytes can be observed in patients with cirrhosis. Most of these do not alter red blood cell function or lifespan. An important exception to this rule is spur cell anemia (SCA), an acquired form of non-autoimmune hemolytic anemia (NAHA) that occurs in advanced liver disease. SCA was first described in 1964 by Smith et al. in a patient with alcoholic cirrhosis who presented with severe hemolytic anemia characterized by bizarre erythrocytes with “curious projections” from their surfaces [1]. In SCA, the shortened life span of the dysmorphic erythrocytes causes anemia that is unresponsive to transfusion. SCA is associated with a poor prognosis and the only known curative treatment is liver transplantation. In this review, we discuss the morphologic abnormalities that are characteristic of SCA and consider theories of pathogenesis, clinical features, and the prognostic significance of SCA, as well as non-transplantation treatments that have been proposed for SCA.
2Illustrative caseA 39-year-old male with a history of heavy alcohol use was found to be anemic (hemoglobin 7.7 g/dL) with elevated liver enzymes (aspartate aminotransferase 109 U/L, alanine aminotransferase 33 U/L) and bilirubin (total bilirubin 10.4 mg/dL, direct 2.2 mg/dL) when he sought medical care for a large bruise on his left leg caused by a fall. A direct antiglobulin test was negative. He was evaluated by a hematologist who suspected that the anemia and indirect hyperbilirubinemia were due to the combined effects of heavy alcohol use and the large hematoma. When the abnormalities persisted despite three months of abstinence and resolution of the hematoma, the patient was referred to a tertiary care center.
At his initial liver clinic visit, the patient was anemic with a marked macrocytosis (hemoglobin 10.6 g/dL, mean cell volume 113 fL) and indirect hyperbilirubinemia (total bilirubin 8.9 mg/dL, direct bilirubin 2.4 mg/dL). Peripheral smear showed acanthocytes, target cells and teardrop cells. Reticulocytes were elevated at 4.6%. The synthetic hepatic function was preserved (albumin 3.5 g/dL), but haptoglobin was undetectable (<10 mg/dL) with a raised lactate dehydrogenase (LDH) (279 U/L). His lipids were elevated: total cholesterol 292 (200-240) mg/dL, high-density lipoprotein 84 (>40) mg/dL, low-density lipoprotein 177 (<130) mg/dL and triglycerides 153 (0-150) mg/dL. Transferrin saturation and ferritin were raised at 84% and 817 ng/mL, respectively. Ultrasound showed a cirrhotic-appearing liver with signs of portal hypertension. He was diagnosed with alcohol-associated cirrhosis complicated by spur cell anemia (SCA) and referred for evaluation for liver transplantation. Atorvastatin 10 mg daily and pentoxifylline 400 mg thrice a day were prescribed for SCA. He did not tolerate pentoxifylline but took the statin for five months, at which time his cholesterol had decreased significantly, but with no improvement in the hemolysis (Table 1), so atorvastatin was stopped.
Comparative laboratory values of the illustrative patient at initial presentation and at presentation six months later with refractory transfusion anemia.
His subsequent course was complicated by hepatic encephalopathy, ascites, hepatic hydrothorax, spontaneous bacterial peritonitis, and coagulopathy. Six months after his initial evaluation in the liver clinic, his ferritin had increased further to 1,461 ng/mL. During admission for gastrointestinal bleeding from a Mallory-Weiss tear, his hemoglobin continued to drop despite transfusion of 3 units of packed red blood cells (PRBC) and resolution of melena. Over the following three weeks, he required an additional 11 units of PRBC in the absence of signs of recurrent bleeding, and his bilirubin rose to 25.2 mg/dL (direct bilirubin 4 mg/dL), reticulocytes to 17.7%, and LDH to 605 U/L. Shortly thereafter, he underwent a successful liver transplant. His explant showed minimally active cirrhosis without steatosis and 4+ hepatocellular iron deposition with an acinar gradient. Within six months of his transplant, his serum iron studies were within the normal range.
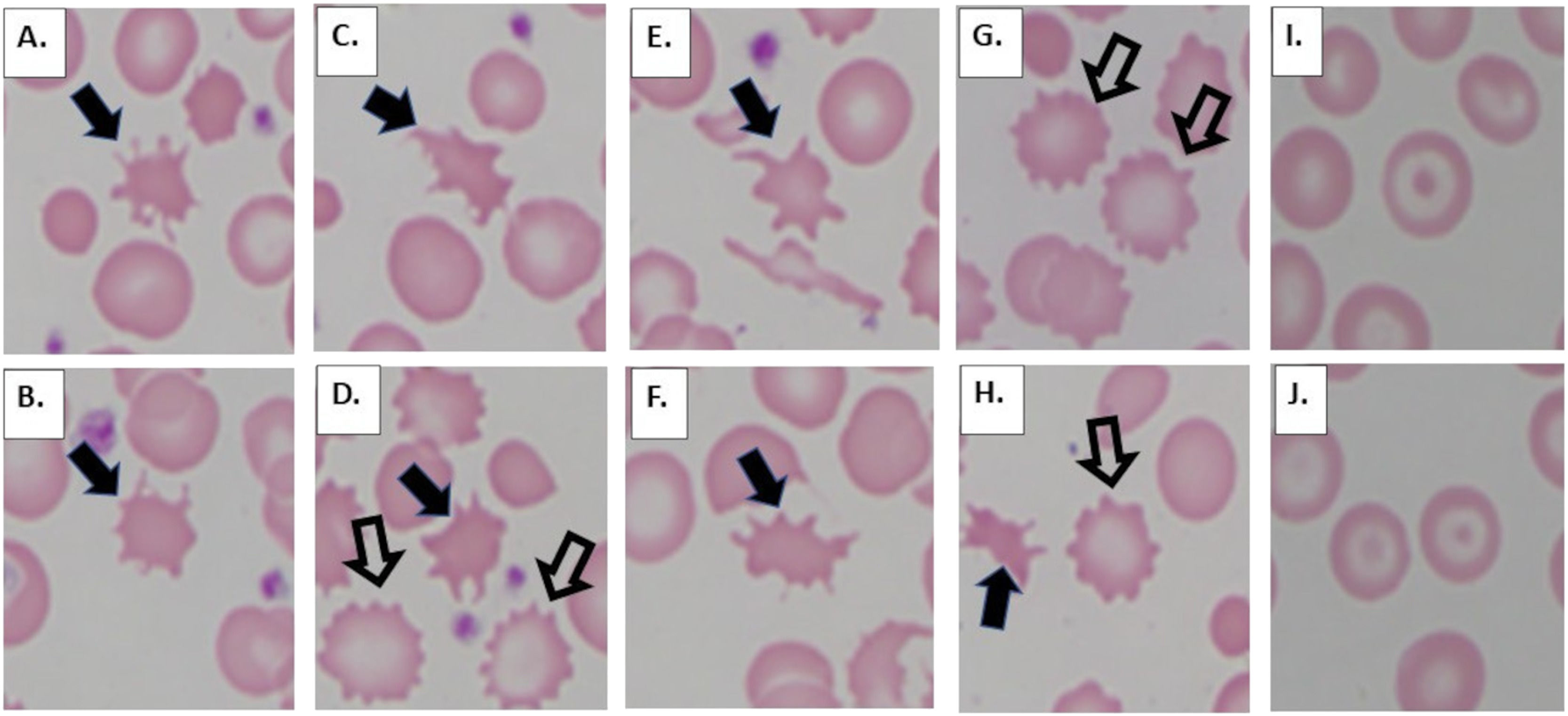
3Review of literature3.1MorphologyAcanthocytes (from the Greek word acantha, meaning "thorn," also known as spur cells) and echinocytes (from the Greek word echinos meaning "sea urchin," also known as burr cells), although often confused with one another, are two different red cell morphologies that are associated with different pathophysiologies [2]. Acanthocytes (Fig. 1 A-F) are red blood cells that have spicules (projections) of varying lengths distributed irregularly on the surface of the cell. Acanthocytes also have a dense center (no central pallor). This is in contrast to echinocytes (Fig. 1 D, G, H), which are red blood cells with short, evenly spaced spicules ("ruffled border") and preserved central pallor. In addition to liver disease, acanthocytes may be seen in patients with abetalipoproteinemia as well as neuroacanthocytosis (choreo-acanthocytosis syndrome and McLeod syndrome). Echinocytes are often artifactual, resulting from prolonged exposure to ethylenediaminetetraacetic acid prior to smear preparation, but maybe physiologic, commonly occurring in association with renal disease/uremia. Codocytes (also known as "target cells") are another red cell morphology that is associated with liver disease (as well as thalassemia and hemoglobin C disease). Codocytes (Fig. 1 I, J) are caused by the redundancy of the red cell membrane and have a bullseye (target-like) appearance. All three of these red cell morphologies (acanthocytes, echinocytes, and codocytes), in addition to several other red blood cell forms, may also be seen in hyposplenism.
Abnormal red blood cell morphologies associated with advanced liver disease. Acanthocytes (A-F, H, solid arrows) are red blood cells with no central pallor displaying relatively long projections which show uneven distribution across the cell surface. Echinocytes (D, G, H, open arrows) are red blood cells with central pallor and short projections which are evenly distributed on the cell surface, forming a "ruffled border." Codocytes (I, J) are red blood cells with a "bullseye," target-like appearance.
First, the limitations of the literature concerning the pathogenesis of SCA must be acknowledged. Most studies on this topic are based on quite small numbers of subjects and some lack cirrhotic controls without SCA. Further, the most recent scientific paper addressing the pathogenesis of SCA was published nearly 30 years ago, and most of the work on this topic is still older. This complicates the interpretation of the research since the terms "burr cell" and "spur cell" were used interchangeably at times in the past [2, 3].
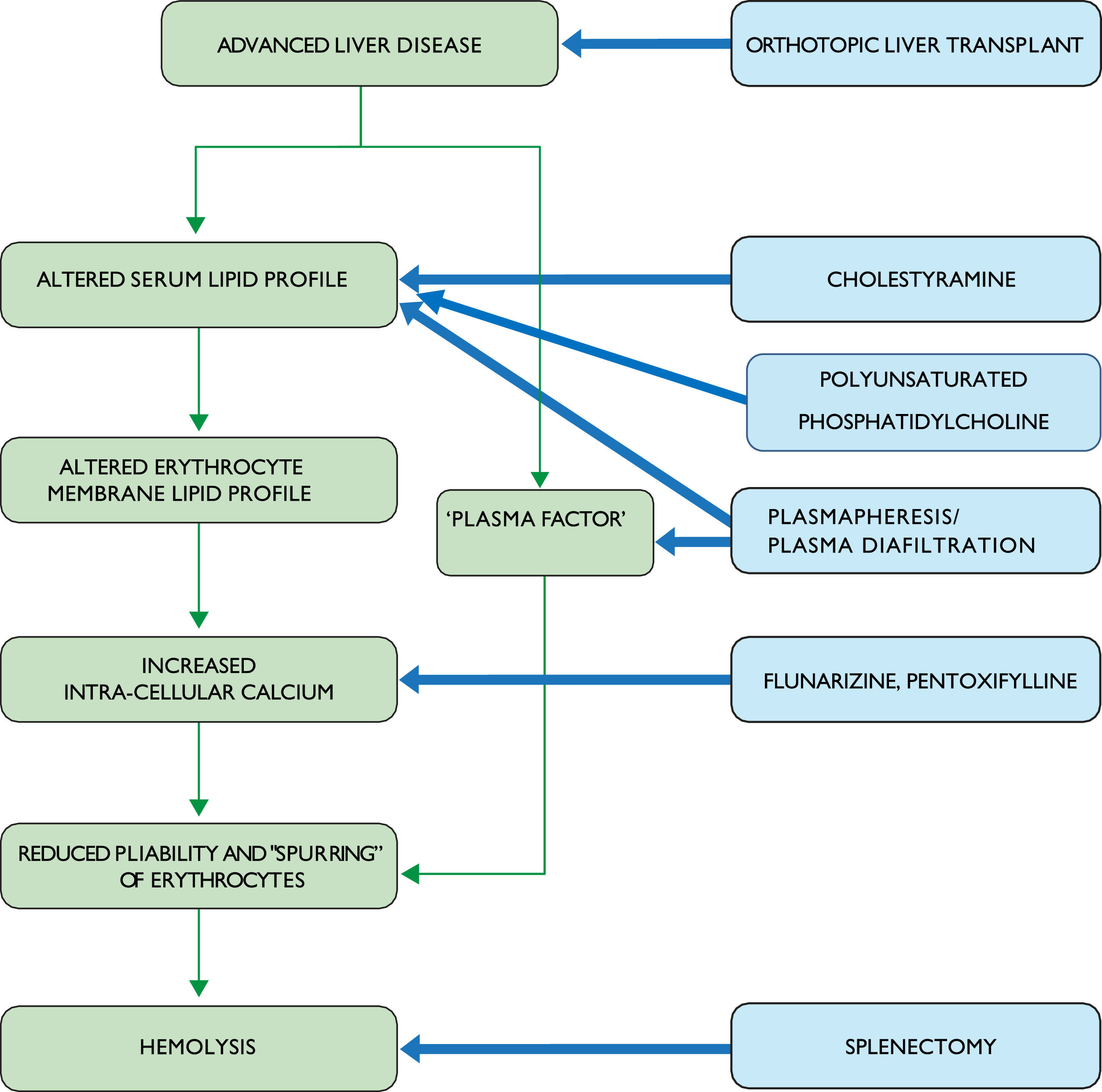
From the beginning, attention focused on abnormalities of lipid metabolism in SCA patients. For context, Zieve's description of hemolysis in hyperlipidemic patients with alcoholic cirrhosis had appeared only a few years before the publication of Smith's case [4]. Like Zieve's subjects, the index SCA patient had remarkably high serum cholesterol (530 mg/dL). However, unlike Zieve's patients, whose hemolysis was mild, transient, and associated with spherocytes, the patient with spurred erythrocytes had unrelenting hemolysis that culminated in his death. Smith observed that the SCA patient's erythrocytes had an elevated cholesterol content and that incubation of normal red blood cells in his plasma increased the cholesterol content of the normal red cells and caused them to develop spiculations. However, the increment in cholesterol content did not invariably precede the spiculations, suggesting that the former was not necessarily the cause of the latter. Based on these observations, Smith proposed that a 'plasma factor' in the serum of the SCA patient, perhaps a protein or a compound bound to a protein, was responsible for the abnormal red cell morphology [1].
Soon thereafter, Silber et al. reported a patient with a very similar presentation; however, neither her serum nor her erythrocytes had elevated cholesterol content and incubation of normal red cells in her serum failed to induce spurring [5]. The claim that serum from SCA patients induces spurring of normal erythrocytes in vitro continues to be cited, but the evidence on this point is problematic, as discussed in this section below. Nonetheless, this phenomenon does appear to occur in vivo, with normal erythrocytes becoming spiculated and undergoing rapid clearance from the circulation following transfusion into patients with SCA, thus accounting for the poor response to transfusion [1, 5].
In a series of influential papers, Cooper promoted the concept that increased red cell cholesterol content is a key pathogenic feature in patients with SCA. This abnormality was attributed to an elevated ratio of free cholesterol-phospholipid in the serum, resulting from reduced activity of lecithin: cholesterol acyltransferase (LCAT), the enzyme that esterifies plasma-free cholesterol [6–9]. The theory postulated that following passive modification of red blood cell (RBC) lipids in response to altered serum lipids, portions of the red cell membrane are removed during splenic transit, leading to spurring, increased membrane rigidity, and accelerated destruction of the cells by macrophages in the spleen. Hypersplenism is presumed to be a critical factor in the resulting hemolytic process since acanthocytes are not associated with hemolysis in the other (non-liver-related) disorders in which they occur [2, 10].
The concept that excess cholesterol is the fundamental defect driving the pathogenesis of SCA remains established dogma to this day [11], notwithstanding that this claim has not been revisited experimentally in 60+ years. In that interval, considerable data have accumulated that raise questions about the relationship of the putative causal abnormalities of lipid metabolism to SCA. In a small series, Duhamel et al. found no difference in total cholesterol levels among cirrhotics with or without SCA and healthy controls. They also found that levels of free cholesterol did not differ between cirrhotics with or without spur cells [12]. Review of SCA cases reported over the past two decades demonstrates that cholesterol levels vary widely among patients with this condition (Table 2). Two recent studies also failed to demonstrate an association between hypercholesterolemia and spur cells. In a study by Vassiliadis and colleagues, cholesterol levels were significantly lower in cirrhotic patients with spur cells than cirrhotics without spur cells [13], while Alexopoulou et al. found that cholesterol levels did not distinguish between cirrhotics with no or low levels of (<4%) spur cells versus those with >5% spur cells [14]. These findings are in keeping with the observation that advanced liver disease is usually associated with reduced cholesterol levels [15, 16]. The patient in our illustrative case initially had elevated cholesterol levels, which fell after several months on a low dose of a statin. Whether the decline in his cholesterol levels was the result of the medication or worsening liver function cannot be determined, but his hemolysis clearly worsened despite the lowering of his cholesterol levels (Table 1).
Lipid levels in cases of spur cell anemia reported since 2000 identified via PubMed.
| Case | Sex | Age | Etiology | Lipids | Reference |
|---|---|---|---|---|---|
| 1 | Male | 30 | ALD | Total chol 386 mg/dLPL 322 mg/dL | [46] |
| 2 | Male | 61 | NASH | Total chol 135 mg/dLHDL 29 mg/dLTG 48 mg/dL, PL 239 mg/dL | [47] |
| 3 | Female | 17 | Post OLT for biliary atresia | Total chol 317 mg/dL | [34] |
| 4 | Female | 61 | PBC | Total chol 209 mg/dLHDL 22 mg/dL, LDL 279 mg/dLTG 120mg/dL | [48] |
| 5 | Female | 32 | ALD | Total chol 97 mg/dLHDL 51 mg/dL, LDL 34 mg/dLTG 61 mg/dL | [49] |
| 6 | Male | 44 | ALD | Total chol 124 mg/dLHDL 21 mg/dL, LDL 94 mg/dLTG 45 mg/dL | [50] |
| 7 | Male | 52 | ALD | Total chol 121mg/dLHDL 15 mg/dL, LDL 29 mg/dLTG 53 mg/dL, PL 112 mg/dL | [40] |
| 8 | Males (5) | 31-49 | ALD | Total chol 38 (34-63) mg/dLHDL 10.7 (5-12) mg/dL,LDL 10 (5.7-19) mg/dLTG 34 (30-51) mg/dL† | [51] |
| 9 | Male | 68 | ALD | Total chol 94 mg/dLHDL 6 mg/dL, LDL 73 mg/dLTG 77 mg/dL | [52] |
| 10 | Male | 26 | ALD | Total chol 215 mg/dLHDL 39 mg/dL, LDL 56 mg/dLTG 84 mg/dL | [41] |
| 11 | Female | 57 | ALD | Total chol 257 mg/dLHDL 46 mg/dL, LDL 193 mg/dLTG 76 mg/dL | [53] |
Chol, cholesterol; ALD, Alcoholic liver disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OLT, Orthotopic liver transplant; PBC, Primary biliary cholangitis; PL, phospholipid; NASH, Non-alcoholic steatohepatitis; TG, Triglycerides.
A second element of the cholesterol hypothesis that is difficult to reconcile with the data concerns reduced LCAT activity. Decreased LCAT activity has been reported in severe acute liver injury as well as advanced cirrhosis [17] and reductions in LCAT activity correlate with impaired hepatic synthetic function in several studies [18–20]. This suggests that reduced LCAT activity is sufficiently common in patients with advanced liver disease that a specific relationship with SCA seems unlikely.
Misidentification of spiculated RBCs appears to have also impeded progress in understanding SCA. In the early 1970s, Brecher and Bessis noted that echinocytes and acanthocytes were often conflated [2]. More than a decade later, Owen et al. asserted that photographs in older studies depicting normal erythrocytes transformed by incubation in serum from SCA patients were images of burr cells, not spur cells [21]. Using electron microscopy, these investigators confirmed that normal RBCs acquire burr cell morphology following incubation in serum from patients with liver disease, even when the serum was from SCA patients. They went on to show that the formation of burr cells involves the occupation of specific binding sites by abnormal high-density lipoprotein and that the process is independent of cholesterol [21]. The discrepancies between these observations and those of earlier studies have not been addressed, much less resolved, but clearly raise questions about the conventional account of the pathogenesis of SCA. The significance of the echinocytogenic property of serum from cirrhotic patients is unknown. Burr cells are not specifically associated with liver disease, although they are sometimes seen in patients with liver disease, with or without SCA (unpublished observations). Some investigators have proposed that burr cells may be precursors of spur cells, but this remains speculative.
Other pathogenic mechanisms have been proposed for SCA. Lithocholic acid, which is formed from chenodeoxycholic acid (CDCA) by the action of intestinal bacteria, has been shown to induce the formation of spur cells in animal models. Cooper and colleagues found elevated serum levels of CDCA in patients with SCA and speculated that this might result in increased production of lithocholic acid [8]. However, they found no measurable lithocholic acid in their SCA patients and increases in conjugated CDCA have been shown in patients with various liver diseases, demonstrating the lack of specificity between this finding and SCA [8, 22, 23]. Allen and Manning reported that RBC phospholipid biosynthesis is impaired in SCA and proposed that abnormalities of this process may contribute to the changes in RBC lipid content [24], but this finding is unconfirmed.
3.3Clinical features and diagnosisThe diagnostic features of SCA are the laboratory findings of hemolysis and the presence of spur cells on peripheral smear. In cases of advanced SCA, up to 80-90% of red blood cells may be acanthocytes. A cut-off >20% spur cells was arbitrarily chosen for the diagnosis of SCA in the past [8], but a lower cut-off has been proposed in recent studies [13, 14].
SCA typically occurs in the setting of advanced liver disease and has been reported in cirrhosis of diverse etiologies, including relatively rare disorders such as familial intrahepatic cholestasis and Wilson disease [25, 26]. However, patients with alcoholic liver disease comprise the majority of reported cases (e.g., Table 2). Interestingly, patients with other common causes of cirrhosis, such as chronic viral hepatitis, accounted for more than half of the cases of SCA in recent studies that used more expansive criteria for SCA [13, 14]. Whether patients with alcoholic liver disease (ALD) are more likely to have a florid presentation of SCA with a large number of spur cells, or whether SCA is more commonly diagnosed in patients with ALD because of the perceived association of SCA with ALD is unknown.
3.4Significance in liver diseaseThe prevalence and overall impact of SCA on the prognosis of patients with liver disease have not been extensively studied. SCA has long been associated with poor survival, but this impression was based on case reports. However, a recent retrospective study confirmed this impression, demonstrating that the 90-day mortality of patients with decompensated cirrhosis and SCA was significantly higher than predicted by those patients' model for end-stage liver disease-sodium (MELD-Na) scores [27]. Furthermore, two recent prospective studies suggest that the impact of SCA on outcomes of patients with cirrhosis may be greater than is commonly recognized. Using the diagnostic criteria of >5% spur cells on peripheral smear, hemoglobin <10 g/dL, and evidence of hemolysis, Vassiliadis et al. reported that 9 of 54 patients (16.7%) hospitalized with advanced cirrhosis and a Child-Pugh score >7 had SCA [13]. Alexopoulou demonstrated a prevalence of 31% (36/116) in hospitalized cirrhotics using the same criteria [14]. The presence of >5% spur cells was associated with more advanced liver disease, higher model for end-stage liver disease (MELD) score and reticulocyte counts compared to patients without spur cells or to those with 1-4% spur cells. Five percent or more spur cells on peripheral smear was also an independent predictor of worse survival after adjustment for age, gender, MELD score, total bilirubin, albumin, prothrombin time, and creatinine. Even with this lower cut-off, outcomes of patients with SCA in these studies were poor, with median survival on the order of 2 months or less.
Recently, Tariq et al. used the Nationwide Inpatient Sample database to assess the impact of NAHA in patients hospitalized with alcoholic liver disease [28]. They found a prevalence of NAHA (mostly attributed to Zieve's syndrome and SCA) of 0.17% in this group. Although the study was limited by administrative coding errors and selection bias, it demonstrated significantly longer hospital stays and higher mortality in patients with NAHA than in those without.
There are no data concerning the prevalence of SCA in outpatients with advanced liver disease, but our experience suggests that this condition may be overlooked in patients who are not decompensated. As seen in our illustrative case report, SCA can present prior to the development of decompensated liver disease. Had it not been for the persistent indirect hyperbilirubinemia in our patient, the diagnosis of SCA might have been missed or at least delayed until he developed a transfusion requirement. When this occurred some months later, he had begun to decompensate relatively rapidly despite abstinence. Presumably, the SCA played a role in driving his decompensation, but the means by which SCA contributes to worsening liver function and poor outcomes remain to be determined.
3.5TreatmentOrthotopic liver transplant (OLT) is the only intervention that has been shown to cure SCA [27, 29-33]. Spur cells disappear from circulation within days of OLT, and SCA patients who undergo OLT have a good prognosis. Interestingly, SCA has been reported to recur after graft failure [30, 34]. Supportive measures that are commonly used in advanced liver disease do not appear to alter the course of SCA and, as our patient demonstrated, SCA does not improve with cessation of alcohol use. The transjugular intrahepatic portosystemic shunt has been reported to have no effect on the degree of hemolysis or percentage of spur cells [30]. Due to severe anemia, SCA patients often receive blood transfusions. Alexopoulou et al. recommended avoiding or at least minimizing blood transfusions in these subjects as they are of limited benefit (due to the acquisition of morphologic abnormalities and shortened survival of transfused red cells) and may contribute to iron overload [14]. This concern is supported by the findings in our patient and by the observations of Pascoe et al., who found heavy iron deposition unrelated to HFE-linked hemochromatosis in explanted livers from patients with ALD and SCA, most of whom had received multiple transfusions [35]. Indeed, Pascoe and colleagues maintain that SCA is the cause of iron overload in cirrhotic patients who lack hemochromatosis mutations [36]. This proposition deserves further study since reports of SCA often describe elevated serum iron tests and/or hepatic iron deposition, and several reports of SCA that predated the discovery of the HFE mutations attributed the underlying liver disease to hemochromatosis [30,37,38].
Given the severity of this disorder and its poor prognosis, various interventions have been tried for SCA (Fig. 2). These invariably involve small numbers of patients, limiting their generalizability. For example, splenectomy has been reported in 2 SCA patients [5, 9]. Both patients experienced massive bleeding postoperatively and died, despite an apparent reduction in the rate of hemolysis. While these reports date back many years, splenectomy remains a high-risk undertaking in the setting of advanced liver disease. Furthermore, a case in which SCA developed eight years after splenectomy indicates that the spleen may be dispensable for the development of SCA [39]. Thus, a splenectomy cannot be recommended as a treatment for SCA.
Other treatment strategies have included plasmapheresis or plasma diafiltration. The rationale for these interventions is that they may reduce or eliminate the "plasma factor" believed to cause spurring. Results have been inconsistent: Cooper's patient showed no improvement in red cell morphology or reticulocyte count after plasmapheresis [9], while Miki et al. found that two rounds of plasmapheresis reduced reticulocyte counts with no change in the percentage of spur cells and no improvement in other markers of hemolysis [40]. Miwa et al. reported that an SCA patient who presented with profound anemia was alive a year after treatment with a combination of supportive measures, including transfusion and nutritional support, combined with plasma diafiltration (a blood purification system that combines dialysis with plasma exchange, which has been studied for removal of water-soluble and albumin-bound toxins in patients with acute liver failure) [41,42]. However, there was no improvement in the percentage of spur cells or his MELD score. In an effort to modulate red cell membrane lipid composition, Salvioli and colleagues gave intravenous infusions of polyunsaturated phosphatidylcholine to 8 SCA patients for five days. Although red cell cholesterol/phospholipid ratios decreased significantly following this treatment, the observed reductions in the proportion of spur cells and unconjugated bilirubin were not significant and no data were provided regarding changes in hemoglobin levels [43].
Another approach has targeted increased intracellular calcium levels in erythrocytes, which cause increased rigidity and susceptibility to hemolysis. Flunarizine is a calcium channel antagonist that has been shown to improve red cell deformability [44]. Fossaluzza and Rossi reported significant improvement in 2 SCA patients treated with flunarizine, but follow-up of these cases was short [45]. Aihara et al. treated an SCA patient with a combination of flunarizine and pentoxifylline, which is well-known for its hemorrheologic effects in peripheral vascular disease [46]. Hemoglobin increased while indirect bilirubin and the percentage of spur cells decreased on this regimen. Cholestyramine was added later based on its lipid-lowering effect and ability to reduce CDCA levels. A year after presentation, the patient was alive and had no spur cells on smear. This is the only recorded case of cure of SCA with medical treatment. While this approach appears promising, flunarizine is not available in the United States and there are no data regarding the use of alternative calcium channel blockers in SCA.
It is clear that the treatments discussed above lack evidence of efficacy, particularly with respect to long-term outcomes, and are largely based on concepts of pathogenesis that have not been validated. They can be considered as temporizing measures while awaiting liver transplantation or as an alternative for patients who do not qualify for liver transplantation, but new approaches to the treatment of this condition are needed.
4ConclusionsOf the various abnormalities of red blood cells in patients with advanced liver disease, SCA is regarded as a rare condition with an ominous prognosis. Recent studies suggest that there is a spectrum of severity of SCA (as assessed by the proportion of spur cells), of which only the most severe cases may be recognized as presentations of SCA. A "milder" form of SCA characterized by fewer acanthocytes is nevertheless associated with hemolysis and poor prognosis as well; this form of SCA appears to be considerably more common than the severe variant. Theories of the pathogenesis of SCA implicate abnormalities of lipid metabolism as the key event in the formation of spur cells, but this understanding is based on small numbers of patients with heterogeneous clinical features and inconsistent use of nomenclature for dysmorphic red blood cells. Abnormalities of lipid metabolism may be necessary but not sufficient to cause SCA.
In summary, SCA is an understudied condition that has significant implications for patient outcomes. Clinicians should consider the possibility of SCA in patients with advanced liver disease, especially those with a disproportionate elevation of indirect bilirubin and/or those whose anemia does not respond appropriately to transfusion. In addition to heightened awareness, there is a need for rigorous, contemporary research into the underlying cause of SCA in order to develop an effective therapy for this condition.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsRuchi Sharma: acquisition of data, analysis and interpretation of data, drafting of the manuscript, final approval of the version to be published; Carol J. Holman: acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, final approval of the version to be published; Kyle E. Brown: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision, final approval of the version to be published. All the authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Declaration of interestNone.
The author's work on this manuscript was supported by their institution.