Due to the high prevalence of recurrent wheezing in the pediatric population, it is important to be able to identify environmental risk factors that may affect the etiology of asthma in several regions.
Objective: to identify possible risk factors associated with asthma in children (9–12 years old) in Passo Fundo, Rio Grande do Sul, Brazil.
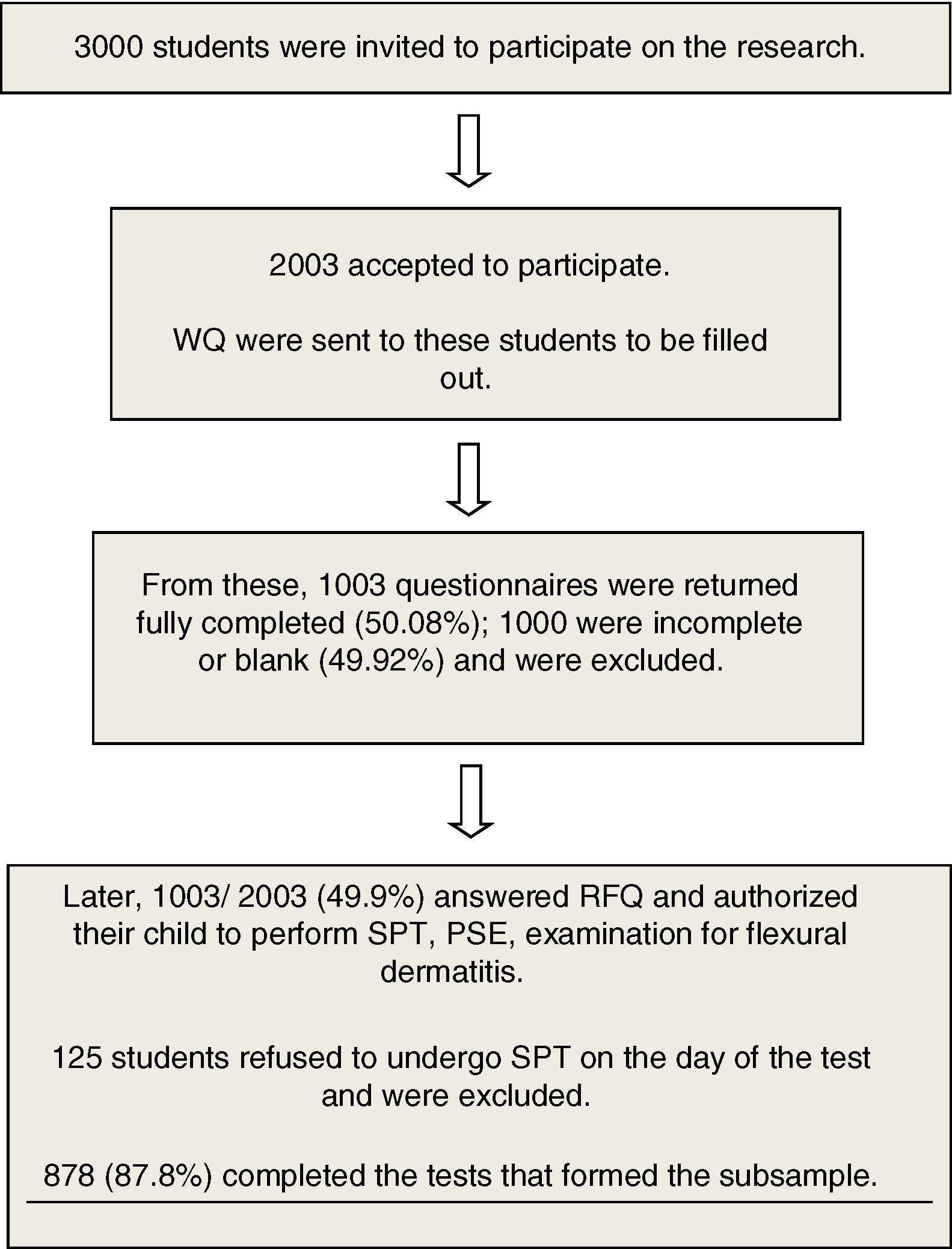
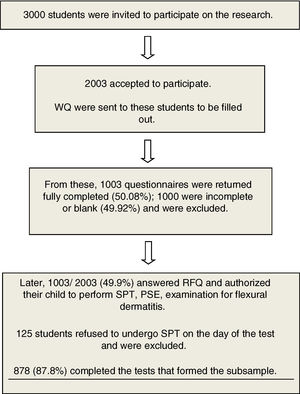
Material and MethodsA total of 1003 school-age children were selected for the cross-sectional study by applying a standardized written questionnaire from the International Study of Asthma and Allergy, and a supplementary questionnaire (ISAAC phase II) was added to address personal, family and environmental factors. Of these, 125 children were excluded because they did not accept to do the skin prick test, resulting in a sample of 878.
ResultsIndependent risk factors associated with asthma were bronchiolitis before two years old [OR]=3.11; 2.23–4.33, current rhinitis [0R]=2.07; 1.43–3.0; sharing bedroom during the first year of life [OR]=2.03; 1.36–3.04; atopy [OR]=1,82; 1.26–2.50; use of paracetamol more than 12 times a year [OR]=1.68; 1.20–2.31; use of antibiotics in the first six months of life [OR]=1,57 1;13–2.17; maternal asthma [OR]=1.75; 1.05–2.78, having an indoor cat during the first year of life [OR]=1.73, 1.07–2.78; premature birth [OR]=1.60,1.02–2.50.
Conclusionour results show that genetic backgrounds, environmental factors, premature birth, use of antibiotics before six months of life, using paracetamol once per month and the presence of co-morbidities such as rhinitis are the risk factors associated with asthma in Brazilian children.
Asthma is a global public health issue, affecting people of all ages.1 According to the Global Initiative for Asthma (GINA) summarized data from both ECRHS and ISAAC appeared in the Global Burden of Asthma report. The report highlighted in 2004 the estimation that as many as 300 million people of all ages suffer from asthma. By 2025, it is expected that this number will rise to 400 million worldwide. The increase in the rate of asthma can be explained by the increase in the number of urbanized communities adopting western lifestyle.2
The majority of countries exhibited an increase in the prevalence of asthma at the end of the last century. However, since 2000, the trends have been different. The prevalence of asthma has increased in some countries such as Italy, Sweden and Denmark, mainly between 2010 and 2014. Populations with a higher prevalence of asthma (>20% in children) are in English-speaking countries and in Latin America.1,2 The prevalence of childhood asthma in Latin America varies substantially (from 4% to 30%) but is above 10% in all the countries.3
Brazil is one of the countries with the highest prevalence of asthma in children, with high rates of severe asthma.4,5 Between 2002 and 2003, 21 centers in Brazil participated in the ISAAC phase III, comprising 58,144 adolescents aged 13–14 years. The mean prevalence of active asthma in this population was 19.0%, ranging from 11.8% to 30.5%.4 The ISAAC study performed in adolescents (13–14 years old) in Passo Fundo (RS) showed that the prevalence for current asthma was 20.5%.6
Alterations in lifestyle could explain the varying rates of asthma in different communities and its growing prevalence worldwide. Asthma, however, is a multifactorial disorder and of genetic susceptibility, with environmental exposure being one of the parties that collaborate for its development. Although environmental factors are generally well established in some populations and regions, studies in regions of variable prevalence are necessary to confirm these factors and identify the differences among regions.
The aim of this study was to investigate the risk factors that may be associated to asthma in schoolchildren living in Passo Fundo (PF), a medium sized city located in the north of the state of Rio Grande do Sul (RS) - Brazil. It shows a temperate climate and good socioeconomic status, with low infant mortality, comparable to developed countries.
Material and methodsStudy population and designThis cross-sectional study was performed in primary schoolchildren in Passo Fundo, located in Southern of Brazil (South latitude 28° 03′ 25″; West longitude 51° 50′ 17); estimated population 200,000 inhabitants [98% in the urban area]; composed by 83% of European immigrants; annual per capita income of approximately US$12,900.00.7 The climate is temperate, annual mean temperature of 17.5°C, and mean air humidity of 70%.
The target population was students aged nine to 12 (about 14,000) enrolled in 75 schools (67 public and eight private ones). Only schools with 50 or more students enrolled in the urban area would be evaluated, thus identifying 47 schools. Of these, 30 were chosen by systematic random sampling, resulting in a sample of 2058 eligible students.
Students’ parents or guardians were invited to participate in the study, and 1003 accepted and answered the written questionnaires in the period within March 2009 and December 2011. The initial questionnaire was ISAAC (WQ), previously validated in Portuguese (Brazilian culture).8–10 After that, the complementary phase IIAC (CQ),11 related to personal, family and environmental risk factors was completed. Among those with valid questionnaires (n=1,003), 878 students performed a prick test for aeroallergens and stool examination, and 125 students refused to do the skin prick test, thus being excluded from the study.
These students had their weight (Uranus UPC 150, Canoas, RS, Brazil), height (Personal Caprice Sanny, São Bernardo do Campo, SP, Brazil) and body mass index (BMI) calculated. All students were submitted to skin prick test (SPT) 13 using standardized aeroallergens (Dermatophagoides pteronyssinus (Dp), Dermatophagoides farinae (Df), Alternaria tenuis (A.tenuis), cat hair, Lolium perenne (Lp) and Barata Cockroach [50% Periplaneta americana, 50% Blatella germanica]), produced by FDA-Allergenic®, Rio Janeiro, Brazil. Histamine (10mg / ml) was used as a positive control and saline as a negative one. The extracts and controls were placed in the volar region of the left forearm, using separate ALK lancets.11,12 Test response was evaluated after 15min; a wheal 3mm or larger in the absence of significant reactivity to the diluent control and positive reaction to histamine was considered a positive reaction. Allergic sensitization was defined as a positive control reaction to at least one of the six allergens applied.11
Three stool samples from all participants were collected on alternate days (stored with modified MIF New Prov; Pinhais, Paraná, Brazil) and stored under refrigeration. Parasitological examination of feces was performed by Hoffman et al. method.13
Fig. 1 shows the flowchart of the study.
DefinitionsCurrent asthma was indicated based on an affirmative answer to the question “Has your child had wheezing or whistling in the chest in the past 12 months?” Active asthma was indicated when it was reported that a child had “Wheezing in the past 12 months plus had asthma ever”. Atopic sensitization was indicated when any of the allergens tested induced wheal with a diameter ≥3mm after subtraction of the negative control. Infection by geohelminths was indicated when parasitological stool examinations showed the presence of their eggs or larvae. Poor neighborhood was classified using the response “the family lives in a suburban area with few parks and gardens”.16 Damp house was indicated based on affirmative answer to the question “does or did your child’s home have damp spots on the wall or ceiling? Living in a street with intense truck traffic was indicated by affirmative answer to the question “How often do trucks pass through the street where you live, on weekdays?”
Statistical analysisStatistical analysis was performed using program SPSS 18.0 (SPSS Inc. 2009. PASW Statistics for Windows, Version 18.0. Chicago, USA). The differences between proportions were assessed by Chi-square test, or Fisher’s exact test. To measure the magnitude of the effect, odds ratio (OR) and 95% confidence interval (95% CI) from simple and multiple logistic regressions were calculated.
The factors identified by univariate analysis with p<0.2 were introduced into the analysis of multivariate logistic regression with the backward stepwise method of variable selection, considering p<0.10 so that the variables would remain in the model. Values of p<0.05 were considered statistically significant.
All parents or guardians provided a signed written informed consent, and this study was approved by the Ethics Research Committee of Federal UFRGS in Porto Alegre, RS, Brazil.
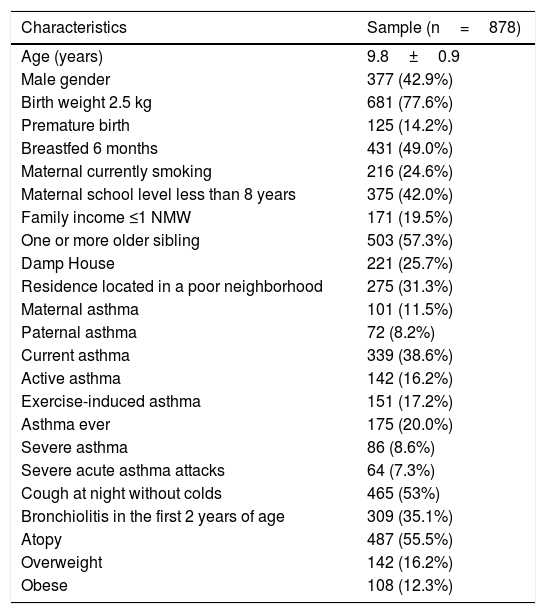
ResultsThe QE and QOL return index was 50.08%, and 878 were submitted to the second phase of the study (TDE, stool parasitological examination, weight and height measures). The prevalence of current asthma was 31.2% and active asthma was 12.4%. Table 1 shows the demography from the total sample (878) that underwent parasitological stool examinations and skin prick tests.
Demographic characteristics of the study population.
| Characteristics | Sample (n=878) |
|---|---|
| Age (years) | 9.8±0.9 |
| Male gender | 377 (42.9%) |
| Birth weight 2.5 kg | 681 (77.6%) |
| Premature birth | 125 (14.2%) |
| Breastfed 6 months | 431 (49.0%) |
| Maternal currently smoking | 216 (24.6%) |
| Maternal school level less than 8 years | 375 (42.0%) |
| Family income ≤1 NMW | 171 (19.5%) |
| One or more older sibling | 503 (57.3%) |
| Damp House | 221 (25.7%) |
| Residence located in a poor neighborhood | 275 (31.3%) |
| Maternal asthma | 101 (11.5%) |
| Paternal asthma | 72 (8.2%) |
| Current asthma | 339 (38.6%) |
| Active asthma | 142 (16.2%) |
| Exercise-induced asthma | 151 (17.2%) |
| Asthma ever | 175 (20.0%) |
| Severe asthma | 86 (8.6%) |
| Severe acute asthma attacks | 64 (7.3%) |
| Cough at night without colds | 465 (53%) |
| Bronchiolitis in the first 2 years of age | 309 (35.1%) |
| Atopy | 487 (55.5%) |
| Overweight | 142 (16.2%) |
| Obese | 108 (12.3%) |
NMW, national minimum wage; NA, not available.
Overweight: 95<BMI>8541.
Obese: BMI ≥9541.
Atopy: NA.
Regarding the prick test, 55.5% of the students were identified as sensitized, regardless of whether they were asthmatic or not (Table 1); of these, 411 (84.4%) were poly-sensitized. The majority of the students with current asthma were sensitized (70.2%) and only 9% were mono-sensitized. Likewise, the majority of the students with active asthma were also sensitized (83.1%, OR=3.16, 95%CI: 4.40–7.60, p<0.001). In addition, students with atopic asthma had more severe asthma when compared to those with non-atopic asthma (OR=2.39; 95%CI: 2.60–7.60, p=0.009). About 77% of students with current asthma had current rhinitis and 47% of those with current rhinitis had current asthma (OR=2.7, 95%CI: 2.0–3.7, p<0.001). Among students with atopic asthma, 95% had positive SPT for mites (D. pteronyssinus and/or D. farinae), 57.5% were positive for L. perenne, 31% for mix of cockroach or cat hair, and 12.8% for A. tenuis.
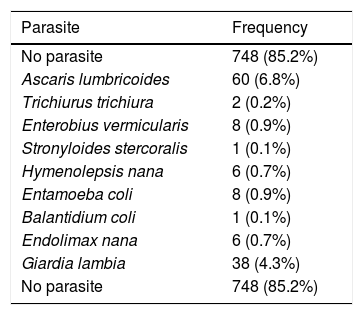
Protozoa or helminths parasites were found in 15% of the students. Those infected with Ascaris lumbricoides had a lower risk of atopy (OR=0.41; 95%CI: 0.23–0.71; p=0.001). Similar results were observed for other helminths (OR=0.56; 95%CI: 0.34–0.91; p=0.016). Table 2 shows the frequency of different species of intestinal parasites found in parasitological stool examinations.
Frequency of different species of intestinal parasites found in parasitological stool examinations.
| Parasite | Frequency |
|---|---|
| No parasite | 748 (85.2%) |
| Ascaris lumbricoides | 60 (6.8%) |
| Trichiurus trichiura | 2 (0.2%) |
| Enterobius vermicularis | 8 (0.9%) |
| Stronyloides stercoralis | 1 (0.1%) |
| Hymenolepsis nana | 6 (0.7%) |
| Entamoeba coli | 8 (0.9%) |
| Balantidium coli | 1 (0.1%) |
| Endolimax nana | 6 (0.7%) |
| Giardia lambia | 38 (4.3%) |
| No parasite | 748 (85.2%) |
Data are presented as n (%), corresponding to the identification of parasites using the method of Hoffman et al.13.
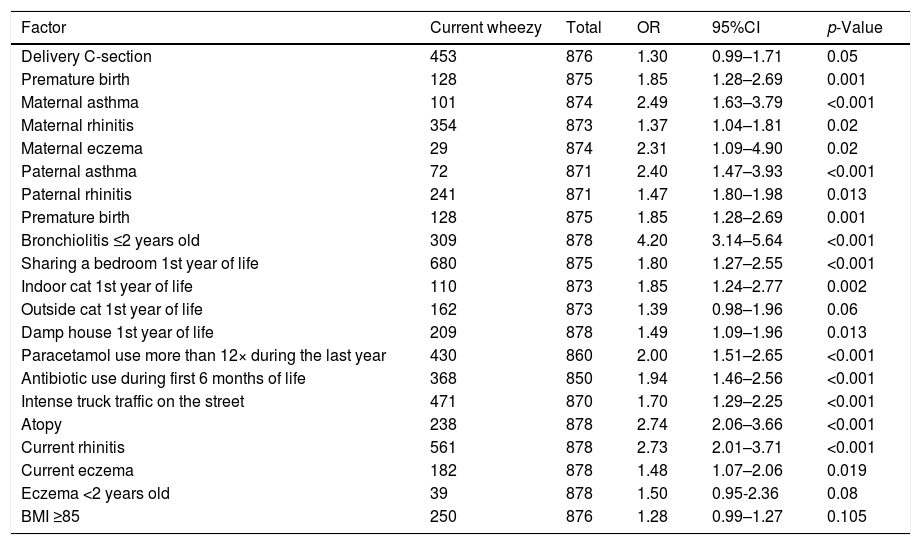
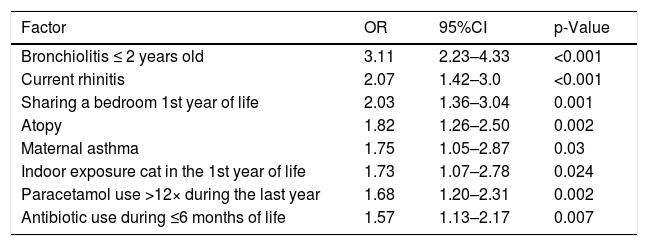
Table 3 shows the significant risk factors associated to asthma after bivariate analysis and Table 4 shows the statistically significant asthma risk factors identified by multivariate analysis (logistic regression).
Risk Factors associated with the presence of current asthma in schoolchildren from 9 to 12 years old in Passo Fundo city: Univariate analysis.
| Factor | Current wheezy | Total | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| Delivery C-section | 453 | 876 | 1.30 | 0.99–1.71 | 0.05 |
| Premature birth | 128 | 875 | 1.85 | 1.28–2.69 | 0.001 |
| Maternal asthma | 101 | 874 | 2.49 | 1.63–3.79 | <0.001 |
| Maternal rhinitis | 354 | 873 | 1.37 | 1.04–1.81 | 0.02 |
| Maternal eczema | 29 | 874 | 2.31 | 1.09–4.90 | 0.02 |
| Paternal asthma | 72 | 871 | 2.40 | 1.47–3.93 | <0.001 |
| Paternal rhinitis | 241 | 871 | 1.47 | 1.80–1.98 | 0.013 |
| Premature birth | 128 | 875 | 1.85 | 1.28–2.69 | 0.001 |
| Bronchiolitis ≤2 years old | 309 | 878 | 4.20 | 3.14–5.64 | <0.001 |
| Sharing a bedroom 1st year of life | 680 | 875 | 1.80 | 1.27–2.55 | <0.001 |
| Indoor cat 1st year of life | 110 | 873 | 1.85 | 1.24–2.77 | 0.002 |
| Outside cat 1st year of life | 162 | 873 | 1.39 | 0.98–1.96 | 0.06 |
| Damp house 1st year of life | 209 | 878 | 1.49 | 1.09–1.96 | 0.013 |
| Paracetamol use more than 12× during the last year | 430 | 860 | 2.00 | 1.51–2.65 | <0.001 |
| Antibiotic use during first 6 months of life | 368 | 850 | 1.94 | 1.46–2.56 | <0.001 |
| Intense truck traffic on the street | 471 | 870 | 1.70 | 1.29–2.25 | <0.001 |
| Atopy | 238 | 878 | 2.74 | 2.06–3.66 | <0.001 |
| Current rhinitis | 561 | 878 | 2.73 | 2.01–3.71 | <0.001 |
| Current eczema | 182 | 878 | 1.48 | 1.07–2.06 | 0.019 |
| Eczema <2 years old | 39 | 878 | 1.50 | 0.95-2.36 | 0.08 |
| BMI ≥85 | 250 | 876 | 1.28 | 0.99–1.27 | 0.105 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; SPT, skin prick test.
Risk Factors associated with the presence of current asthma in schoolchildren from 9 to 12 years old in Passo Fundo city: Logistic regression.
| Factor | OR | 95%CI | p-Value |
|---|---|---|---|
| Bronchiolitis ≤ 2 years old | 3.11 | 2.23–4.33 | <0.001 |
| Current rhinitis | 2.07 | 1.42–3.0 | <0.001 |
| Sharing a bedroom 1st year of life | 2.03 | 1.36–3.04 | 0.001 |
| Atopy | 1.82 | 1.26–2.50 | 0.002 |
| Maternal asthma | 1.75 | 1.05–2.87 | 0.03 |
| Indoor exposure cat in the 1st year of life | 1.73 | 1.07–2.78 | 0.024 |
| Paracetamol use >12× during the last year | 1.68 | 1.20–2.31 | 0.002 |
| Antibiotic use during ≤6 months of life | 1.57 | 1.13–2.17 | 0.007 |
*Statistically significant (p≤0.05).
OR: odds ratio; CI: confidence interval.
Asthma is a syndrome comprising distinct phenotypes (e.g., atopic vs. non-atopic asthma) and various risk factors. Given the size and diversity of Latin America, it is expected to find a variability in the relative importance of each asthma phenotype and its risk factors. Many urban populations in Latin America present a high prevalence of asthma symptoms in childhood. In addition to that, and in contrast to developed countries, asthma in these populations is less significantly associated with atopy. Moreover, a non-atopic phenotype has been described as the most common presentation of childhood asthma in regions such as Brazil.14,15 There is no consensus on why this may occur, but one possible explanation is that the role of crowding in poor urban environments as well as the early introduction of nursing care for children before the age of two may facilitate this early and aggressive milieu with respiratory viruses and other environmental factors.16
A study conducted in an urban center in Southern Brazil demonstrated that current wheezing at 10 years was high (26%), but the vast majority (70%) with current asthma were non-atopic.17 In another study carried out in the city of Sao Paulo with adolescents aging from 13 to 14 years, it was found that 49.4% of adolescents with current asthma were sensitized to aeroallergens,18 corroborating with the findings of this study, where we identified 55.5% of schoolchildren as atopic, regardless of whether or not they were asthmatic. And among asthmatics, 70.2% were atopic and poly-sensitized.
The majority of the population studied in Latin America within the framework of the ISAAC are of low socioeconomic level and are exposed to high levels of intestinal helminths and viral infection. Paradoxically, these factors may inhibit or stimulate allergic and airway diseases in this setting.14,16,19 In the present study, protozoa or helminth parasites were found in 15% of the students. Those infected by Ascaris lumbricoides had a lower risk of atopy. Studies have shown that heavy infection by ascaris and trichuria during childhood has been shown to reduce the risk of school-age atopy among Brazilians.20
Recently, a study has shown that atopic sensitization related to current wheezing symptoms is strongly associated with per capita national income. Regions with per capita income equal to or greater than $9,200 showed a stronger association between asthma and a positive SPT.21 This gives us the idea that in communities where there is good social development, the high prevalence of asthma symptoms is more associated with atopy, since this association may not be so among communities with low socioeconomic status. In our study, only 20% of the children had a family income ≤1NW (≤US $ 300.00) and there were few children positive to parasitology. This is unlike other studies in Latin America, which evaluated children with lower income and higher rates of helminths in parasitological tests.14,17
Respiratory viral infections, mainly due to respiratory syncytial virus (RSV), commonly associated with acute bronchiolitis in the first two years of life, have been associated with asthma in developing countries. Factors virus in combination with genetic predisposition of hosts mediating the relationship between viral respiratory infections and asthma.16,22 A recent study in children in a poor urban community of Southern Brazil showed that early history of bronchiolitis was the leading independent risk factor for asthma at 10 years of age.17 Another study in Southern Brazil showed early-life bronchiolitis was highly associated with current asthma in children four to five years of age.22 In our study, an episode of bronchiolitis in the first two years was a strong independent risk factor for asthma. Thus, the findings suggest that early exposure to viral infections, in particular respiratory syncytial virus, appears to be a significant risk factor associated to asthma in Latin America.
Exposure to animals inside the house as either a risk or protection factor for asthma has been a subject of controversy. Early exposure to cats in the house, mainly to atopic high risk children was identified as a risk for asthma, and for more severe asthma.23,24 In our study, exposure to cats over the first year of life, but not to dogs, was significantly associated with current asthma, the same was found in the study by Ganesa et al.25
Epidemiological studies have consistently shown that asthma and rhinitis are strongly associated and that allergic rhinitis is a risk factor for the development of asthma,26 and if associated with atopic dermatitis the risk is significantly increased.27 - In the present study, having current rhinitis doubled the risk of having asthma.
Premature birth is another factor that has been associated with the symptoms of asthma and other pulmonary diseases.28 As observed by other authors,29 premature birth, and not low birth weight, is significantly associated with a greater risk for asthma.
To date, reviews of studies evaluating the relationship between breastfeeding and wheezing show that there is no statistical evidence to support the protective effect of breastfeeding on the development of wheezing, atopy or eczema in young infants.30,31 In the present study, a relationship between breastfeeding and the development of wheezing was not identified.
Studies have shown an association between paracetamol use and asthma. Frequent use of paracetamol may influence the development of asthma by reducing the levels of glutathione in the mucosa of inferior airways, shifting the oxidative/non-oxidative balance toward more oxidative events, and favoring the inflammatory process at these sites.32–34 In the present study there was a strong association with paracetamol used more than 12 times/year and current asthma. However, these data must be interpreted cautiously, because other painkillers and anti-inflammatory medications were not investigated.
The use of antibiotics in the first months of life has been associated with early wheezing and asthma. A suggested mechanism for this association is the immunological stimulation that originates from changes in the intestinal flora.34,35 Recent studies have shown an association between asthma and use of antibiotics,36,37 especially if used before two years of age. A study published in 2018 found that antibiotic use in the first week of life was associated with an increased risk of atopic asthma at 12 years.38 In the present study, an association between asthma and antibiotic use was seen only in children who took antibiotics in the first six months of life.
The hygiene hypothesis suggests a protective effect of large families, with many children living in the same environment and often sharing the same bed. This environment favors viral infections and may increase the exposure to endotoxins, and these factors may protect the child from sensitization to aeroallergens, asthma, and rhinitis.39 On the other hand, in our study, sharing a bedroom during the first year of life was strongly associated with asthma (OR=1.80).
The present investigation has limitations. It was a cross-sectional study performed with questionnaires that were answered by parents or guardians in their homes, which could have led to memory or confusion bias. For some parents, their ability to interpret the questions may have been limited, because 40% reported having up to eight years of education, at most, members of this group would have completed primary studies. However, as recommended by the ISAAC project, our sample was very comprehensive, reaching practically all socio-economic layer levels. In addition, the distribution of schools was well balanced, reaching several regions of the city.
In conclusion, the prevalence of childhood asthma was high in Passo Fundo city, and the associated risk factors found in this population are in agreement with the current literature. Since most Latin American nations share barriers to asthma management, and given the profound diversity of Latin America, further studies are needed to identify and be able to expose the population of each studied area and the resulting stress in asthma for a better understanding of the etiopathogenesis of asthma in children.
Conflict of interestThe authors have no conflict of interest to declare.
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
The authors state that the project was approved by the Research Ethics Committee of UFRGS, protocol n. 12878.