Hymenoptera venom allergy (HVA) is one of the most frequent causes of anaphylaxis following a bee, vespid or ant sting. Real-life data regarding the management of HVA in children are lacking. To address this unmet need, we carried out a survey defining the current management of HVA in children among pediatric allergists in Italy.
Educational investments on the improvement of the management of pediatric patients with HVA are urgently needed, and our analysis represents a relevant instrument in targeting a roadmap with this aim. The time for pediatric allergists to take action has come, and a task force from the Rare Allergic Diseases Commission of the Italian Society of Pediatric Allergy and Immunology is working on the topic to improve pediatricians’ knowledge and optimize the care of these patients.
Hymenoptera venom allergy (HVA) is a potentially life-threatening allergic reaction following a bee, vespid or ant sting. Clinical manifestations range from local reactions at the sting site to systemic sting reactions (SSR)1. Although the availability of epidemiological data is limited, SSR have been reported in up to 3% of adults 2 and 2.5 % of children 3–5 in accordance with age and nationality.
HVA is an important cause of anaphylaxis, accounting for about one quarter of the cases caused by known allergens6–7. The only current treatment that can potentially prevent further SSR is venom immunotherapy (VIT). VIT results in long-term clinical benefits and improved quality of life in both adults and children8–9. However, despite these possible advantages, VIT is still not commonly used by physicians across all European countries, in particular for pediatric patients10. Furthermore, there is a lack of real-life data regarding management of HVA in children in the scientific literature.
In order to address this unmet health-care need, we carried out a survey, defining current management of HVA in children among physicians belonging to the main paediatric allergy centres in Italy. To the best of our knowledge, our work is the first that attempts to collect real-life data regarding this topic at a national and international level. For our purpose, we organised a dedicated meeting inside the 21st Annual National Congress of the Italian Society of Pediatric Allergy and Immunology, held in Milan from the 16th to the 18th of May 2019.
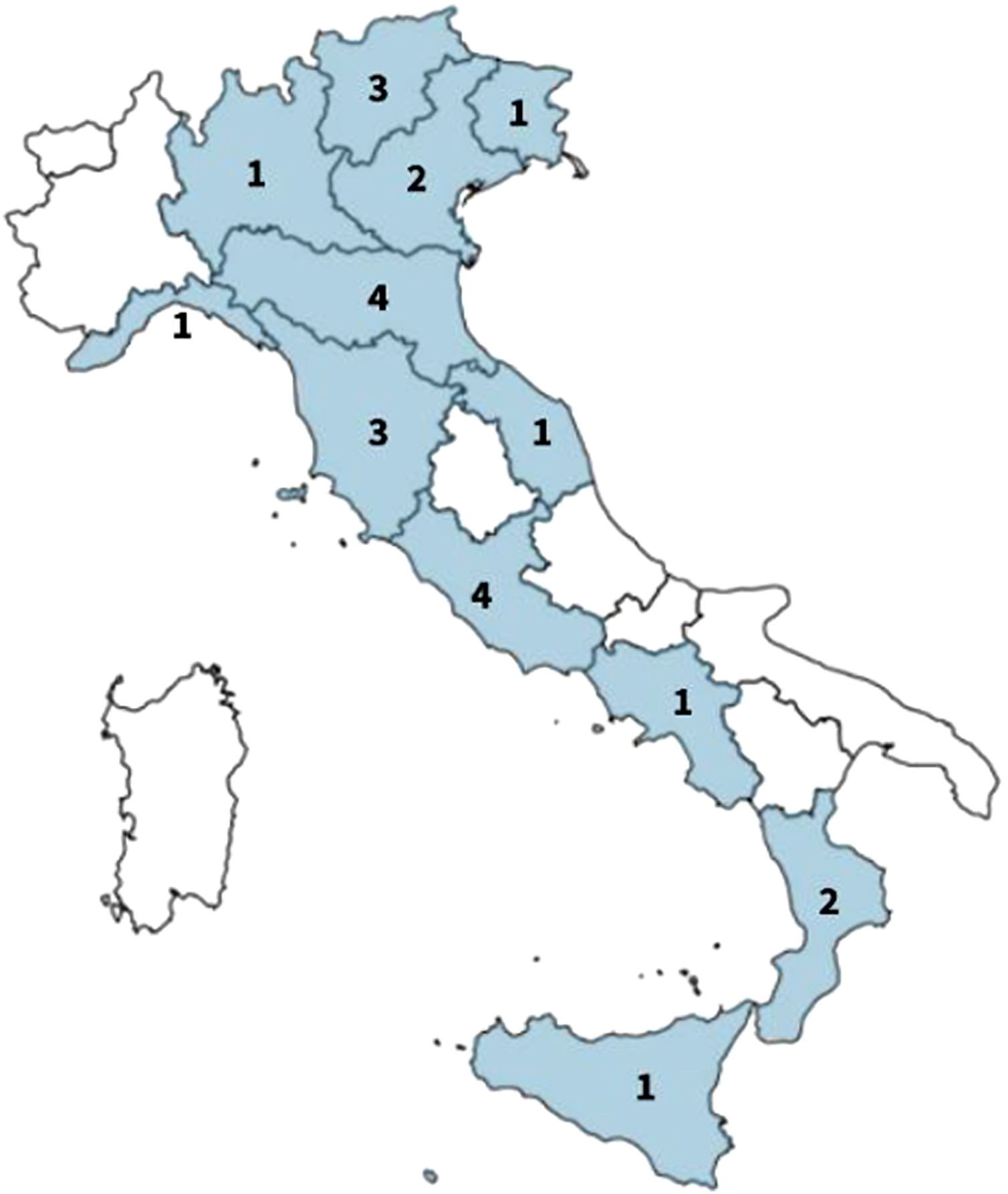
An open invitation to the event was sent to all the main pediatric allergy centres in Italy: 20 centres sent a representative to the meeting to contribute to the survey, four centres whose spokesperson did not have the chance to join the meeting decided to complete the survey remotely in the same timeframe and to send their answers via e-mail. Representatives involved in the survey belonged to 12/20 Italian regions located in different parts of the country, from north to south (Fig. 1).
According to the answers of the representatives (Table 1), only slightly more than half of them evaluate patients with HVA, representing a first specific target to be improved. The vast majority of the centres that did not evaluate them referred patients with HVA to a reference centre, more frequently to an adult one.
Answers to the survey of the representatives of the main Italian pediatric allergy centres.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Approximately half of the centres which evaluated pediatric patients with HVA have more frequently managed > 20 patients so far, pointing out that these centres seem to concentrate the main flux of patients. This fact seems to be rational for a relatively rare clinical condition such as HVA, compared to other prominent pediatric allergic diseases, in order to maximize the cost-benefit ratio in our country, where the health system is public. At least some Italian regions such as Tuscany manage referrals according to the network model with hubs and spokes, in which all the cases are followed in a tertiary hospital with specific expertise on the topic. However, this is not true for all the regions, and it seems that some of them could benefit from the implementation of such a strategy.
An entomological display case is used only in nearly one-third of the centres. Data from the scientific literature highlight how people from the general population11 and allergy specialists12 have a poor performance in insect recognition (excluding the honeybee), making their visual identification unreliable. For this reason, efforts should be made to promote the diffusion in all the centres through entomological display cases or at least high-resolution full-colour pictures of insects, together with active collaboration and training with an expert entomologist. These solutions could be pivotal in sharing information among pediatric allergists and families.
Diagnoses according to current guidelines were made in approximately three out of four centres, and the Italian consensus with practice criteria for the management of HVA1 was the most frequent type of guideline followed. This may also be due to the fact that the latter was primarily written in Italian and, for this reason, accessible to the majority of Italian physicians. However, international guidelines issued by the European Academy of Allergy and Clinical Immunology (EAACI)13 were commonly used as well. Furthermore, it is remarkable how in one centre in the Northern part of Italy, near to the border with Austria, German Society for Allergology and Clinical Immunology guidelines14 were followed, underlining the complex cultural texture of our country.
Slightly more than half of the centres perform VIT, more frequently a full cycle, representing another specific target to be improved. Moreover, slightly more than half of the centres that did not perform this therapy did not refer eligible patients to a reference centre to treat them, and when they did so, the referral was more frequently made to an adult one. Given the potential life-saving role of VIT in eligible patients, increasing the proportion of them who undergo the treatment represents a primary objective.
The centres that performed VIT, including those performing only its maintenance phase, more frequently had managed it in <5 patients so far, in slightly less of one-third of the centres and in two-thirds of the centres, respectively. However, the number of patients appears scarce, and the activity in these centres should be implemented.
The centres also performing the build-up phase of VIT used a traditional protocol more frequently.
The vast majority of all the centres performing VIT adequately monitored the results of specific cutaneous tests and specific immunoglobulin E to venom over the treatment.
Patients who completed the treatment were < 5 in approximately half of the centres performing a full course of VIT and in two thirds of the centres, performing only its maintenance phase. However, the number of patients appears scarce, and the activity in these centres should be implemented as well.
When asked to express their opinion about the main issues associated with the management of pediatric patients with HVA, representatives more frequently expressed that HVA is rare. However, these thoughts, expressing a certain grade of self-confidence, seem to be in apparent discordance with the data emerging about the practical experience of the centres. In addition, the latter appears in noticeable discordance with other pediatric epidemiological data on HVA, including SSR.3–5
Ciccarelli et al.15 have recently published a paper assessing the knowledge of Italian emergency care physicians on HVA. They administered a multi-centric, single-city survey to a small group of doctors working in different emergency departments, finding an unsatisfactory level of knowledge on the topic (e.g. Hymenoptera sting reactions classification, auto-injectable epinephrine prescription, referral to a specialist follow-up, prevention strategies such as VIT), with the exception of its acute management. Moreover, the authors highlighted the wish for better education on the topic, which was expressed by emergency care physicians advocating the need for closer collaboration with allergists. Parallel data on the knowledge of Italian adult or pediatric primary care physicians about HVA are lacking.
To the best of our knowledge, our work is the first at national and international level that tries to collect real-life data regarding the current management of HVA in children among hospital pediatric allergists. The main strength of our work is the number and heterogeneity of representatives involved in the analysis, who belong to various centres located in different parts of the country, from north to south. The main weakness of our work is due to the fact that, even if an open invitation to participate in the survey was sent to all the main pediatric allergy centres in Italy, a few of them did not succeed in taking part in it. However, we are confident that our analysis included almost all of the main centres. Moreover, although the situation in Italy may be similar to that of other European countries, at least in the Mediterranean area, further data should be collected abroad on the topic to compare our findings.
Educational investments to improve the management of pediatric patients with HVA seem of fundamental importance in the agenda, and our analysis represents a relevant instrument in targeting a roadmap with this aim. Indeed, specific pediatric care of children with HVA seems to be preferable when possible. For this reason, the time has come for pediatric allergists to take action in order to increase pediatricians’ knowledge, optimizing the care of these patients.
Confidentiality of dataNot applicable.
Right to privacy and informed consentNot applicable.
Protection of human subjects and animal in researchNot applicable.
FundingNone.
Conflict of interestThe authors have no conflict of interest to declare.
Contributors’ statementMG and EN conceptualized, designed the work, acquired, analyzed the data, drafted the initial manuscript and reviewed the manuscript. RC analyzed the data, drafted the initial manuscript and reviewed the manuscript. FM, SA, SB, FS, CM, LP, LL, LC and GLM analyzed the data and reviewed the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Declaration of originalityThis manuscript has not been published previously and it is not under consideration for publication elsewhere. The publication of this work is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If the manuscript is accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
The authors want to thank the representatives of the Italian pediatric allergy centres who answered to the survey: Fernanda Chiera, Francesco Paravati (Crotone), Tiziana Foti (Reggio Calabria), Guglielmo Scala (Naples), Maurizio Poloni (Rimini), Arianna Giannetti, Giampaolo Ricci (Bologna), Giovanni Cavagni (Parma), Carlo Caffarelli (Parma), Giulia Brindisi, Marzia Duse (Rome), Maria Cristina Artesani, Alessandro Fiocchi (Rome), Anna Maria Bianchi (Rome), Elena Galli (Rome), Maria Angela Tosca (Genoa), Amelia Licari (Pavia), Fabrizio Franceschini (Ancona), Giovanni Battista Pajno (Messina), Sofia D’Elios, Diego Peroni (Pisa), Cinzia Cioni, Roberto Bernardini (Empoli), Lucrezia Sarti (Florence), Manuela Pace, Ugo Pradal (Rovereto), Stefanie Plössel (Silandro), Lydia Pescollderungg (Bolzano), Giorgio Piacentini, Attilio Boner (Verona), Francesca Lazzarotto, Antonella Muraro (Padua).