Respiratory allergies, such as allergic rhinitis and asthma, are a frequent disorder affecting up to 40% of the general population. Allergic disorders are characterised by abnormal production of allergen-specific immunoglobulin-E (IgE). IgE may be easily evaluated at serum level. Thus, serum specific IgE may be considered a biomarker for identifying the allergic phenotype.
Sensitisation is the immune phenomenon defined by the production of allergen-specific IgE. Sensitisation may be demonstrated by positive skin prick test (SPT) or serum IgE measurement. However, sensitisation does not correspond to allergy. In fact, the diagnosis of allergic disorders is based on the demonstration of a cause-effect relationship: such as the occurrence of symptom consequent to the inhalation of the allergen causing sensitisation. In this regard, positive SPT is a necessary, but not sufficient, condition for diagnosing allergy. The diagnostic pathway should consider the history of the individual patient. Therefore, managing allergic patients well is mandatory to optimise medical resources.
Skin prick tests are considered positive when the cutaneous reaction is equal (graded as +++) or more (++++) than that provoked by histamine. Consequently, there are only two grades of cutaneous positivity: +++ or ++++. On the contrary, serum IgE assessment allows a wider scale ranging from 0.35 to >100kU/L. Therefore, the hypothesis is that serum IgE measurement might be more useful than SPT in discriminating sensitisation from true allergy. To test this thinking, we evaluated a group of consecutive subjects reporting respiratory symptoms suggestive for allergy, i.e. nasal itching, sneezing, watery rhinorrhoea, nasal obstruction, occurring after exposure to allergens. All patients referred to the Respiratory Diseases Clinic of the San Matteo Hospital of Pavia (Italy). All patients were visited and SPT was performed according to the EAACI guidelines.1 The procedure was approved by the Review Board and all patients gave a written informed consent.
We considered only patients with SPT 4+, hypothesising that the maximal cutaneous response could be useful to discriminate true allergy. Thus, 122 patients (71 males, mean age 33.2 years) were further studied, also assessing serum specific IgE. Serum-specific IgE were detected by the IFMA procedure (ImmunoCAP Thermo Fisher Scientific) in peripheral blood samples from patients. Quantitative specific IgE concentrations were expressed in kU/L according to the traceable calibration to the 2nd IRP WHO for Human IgE. Specific IgE levels were considered positive over 0.35kU/L.
Statistical analysis was performed using the statistical software package Medcalc 9 (Frank Schoonjans, BE). Medians (md) and percentiles (25th and 75th, IQR) were used as descriptive statistics. The non-parametric Wilcoxon's test was used to compare samples. The non-parametric Kruskal–Wallis rank test was performed to evaluate the analysis of variance between groups of patients. In addition, a receiver operating characteristic (ROC) curve analysis was performed in order to determine a cut-off for sIgE that could optimise the sensitivity and the specificity of the test, to identify patients’ aeroallergens sensitisation or allergy. A p-value ≤0.05 was considered statistically significant.
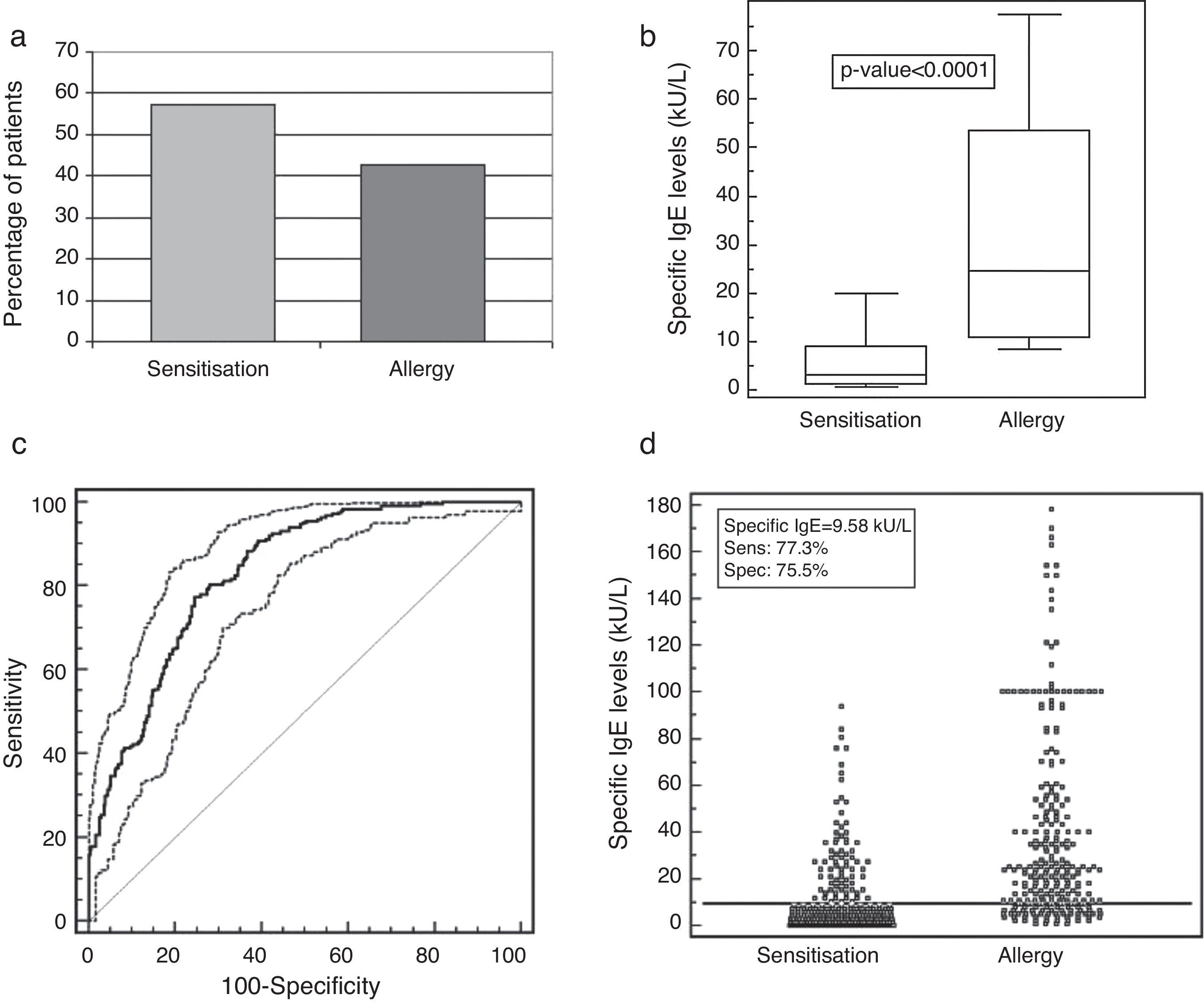
The comparison between history and positive allergy testing revealed that 42.6% of patients were true allergic, while 57.4% were only sensitised, as shown in Fig. 1a. Moreover, serum IgE levels were significantly higher (p<0.0001) in patients with allergy rather than in patients with sensitisation (Fig. 1b). The ROC analysis showed a sIgE concentration >9.58kU/L to be the optimal cut-off to discriminate sensitisation and allergy. The associated sensitivity and specificity were 77.3% (95% CI 71.6–82.3) and 75.5% (95% CI 70.3–80.2), respectively. The positive and negative predictive values were 71.9% and 80.4%. The corresponding area under the ROC curve of 0.831 (95% CI 0.797–0.861) indicated a good discriminating ability (Fig. 1c and d).
(a) Percentage of patients with sensitisation and allergy. (b) Specific IgE distribution (kU/L) evaluated in patients with allergy (causal allergens) or with sensitisation (sensitising allergens) to aeroallergens with skin prick test 4+. Values were represented as medians (black line), quartiles (25th and 75th percentiles, white box), and p-value between the groups. (c) ROC curve and (d) discrimination ability of the chosen cut-off of 9.58kU/L for the specific IgE levels in patients with sensitisation or allergy to aeroallergens with skin prick test 4+.
Therefore, this study, conducted on a real life basis, demonstrates that true allergy is documented in less than 50% of sensitisation. This finding is clinically relevant, overall concerning the decision of prescribing allergen-specific immunotherapy. Therefore, SPT could be considered a first line testing: useful for selecting patients with possible allergy. On the contrary, serum-IgE measurement may be a reliable tool for identifying true allergic patients and choosing the allergen extract for immunotherapy.
Ethical disclosuresProtection of human subjects and animalsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.