Fixed drug eruption (FDE) is a commonly reported adverse drug reaction, characterized by single or multiple round oedematous erythemato-violaceous plaques with well defined borders and often central bullous detachments.1,2 Lesions usually develop 48h after drug intake and recur at the same sites upon reexposure to the offending drug.3,4 Spontaneous resolution with residual hyperpigmented post-inflammatory patches is also characteristic.3,4
Non-steroidal anti inflammatory drugs (NSAIDs), in particular nimesulide and piroxicam, are the most frequent culprit agents, followed by antibiotics and anticonvulsants.1,3,4
Etoricoxib is a recently developed selective cyclo-oxygenase (COX) isoenzyme 2 inhibitor which has rarely been described as a cause of FDE.5–8
We report a case of fixed drug eruption due to etoricoxib. A 38-year-old man with allergic rhinitis and no history of drug allergy was referred to our outpatient clinic due to multiple sharp round erythematous pruriginous patches of diameter 1–4cm on the upper and lower limbs, trunk and genitals. The patient mentioned several exacerbations along the previous year, approximately once a month. In most episodes no triggering factor could be determined but in two occasions he noticed that lesions relapsed 15–30min after intake of etoricoxib, frequently taken by the patient for musculoskeletal pain. There was spontaneous improvement after 3–4 days after stopping NSAIDs intake, but multiple residual hyperpigmented lesions persisted for several months. The patient mentioned regular consumption of paracetamol and ibuprofen for headaches or musculoskeletal pain, apparently with no adverse reaction.
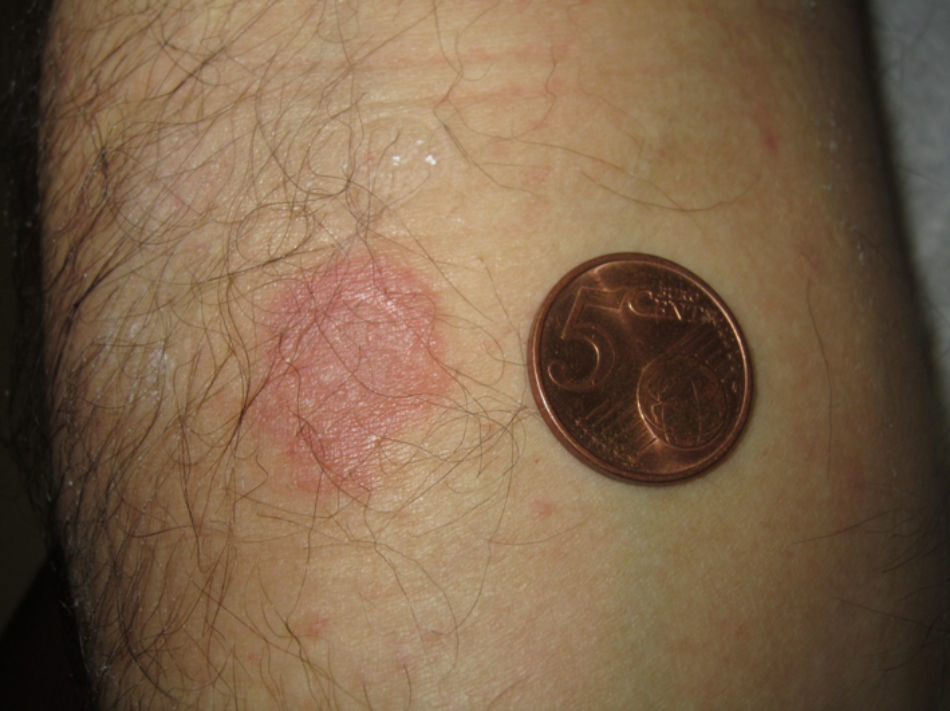
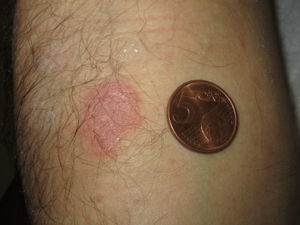
The patient underwent patch testing with paracetamol, ibuprofen, nimesulide and etoricoxib (30% in petrolatum), applied to the hyperpigmented lesion in the right forearm. Patch tests were performed several months after the resolution of the last adverse reaction. An erythematous papular reaction was observed with etoricoxib on the lesional skin 48h and 96h later (Fig. 1). A pruriginous flare in one single residual lesion of the trunk was also reported. The patch tests with the other three drugs were negative.
No flares of FDE occurred after withdrawal of etoricoxib and reexposure to nimesulide, ibuprofen and paracetamol did not cause recurrence of the lesions (self initiative).
In our case, the culprit drug was promptly confirmed by patch testing on residual lesions, as described for several NSAIDs, including etoricoxib.1–4
Despite being a recent drug, etoricoxib is widely used in many countries. Because of its high level of COX2/COX1 selectivity, it combines high anti-inflammatory activity (equivalent or superior to that of conventional NSAIDs) with a lower incidence of side effects.9 The most commonly reported cutaneous reactions due to etoricoxib are urticaria and angioedema followed by sporadic cases of erythema multiforme, Stevens–Johnson syndrome, acute generalized exanthematous pustulosis and rarely FDE.5–10
Patch testing has been widely recommended as the initial diagnostic tool in FDE patients.1–4 This method is useful in the etiologic investigation of FDE and avoids the risks of systemic drug reexposure. Although pure allergens are commercially available for some drugs, others must be prepared by using the powder of commercial tablets in pet (recommended concentrations between 5% and 30%).3 We adopted the maximum recommend concentration (i.e. 30% in petrolatum for commercial tablets) in order to avoid false-negative results.
The rationale for patch testing on lesional skin resides on the activation of intra-epidermal memory T cells by the re-exposure to the offending drug.8 Non-lesional skin patch tests are useful only for control purposes and negative results are expected in the vast majority of patients.1,4 For this reason, we only applied the drugs on lesional skin.
Drug eviction is the mainstay of treatment in non-IgE mediated drug allergy. In our case, as other NSAIDs were available and well tolerated by the patient, we recommended the eviction of all selective COX2 inhibitors. Until now no cross-reactivity has been described between these molecules possibly due to differences in the molecular structure of etoricoxib.5,7,10 However, more data are still required before assuming the absence of cross-reactivity between different COX2 inhibitors.
In this case, as sustained by literature, patch testing was of unquestionable value in the identification of the culprit agent. More research is still required for standardization of this technique, particularly for drugs exclusively available as commercial tablets. Patch testing was also useful in the search for safe alternative drugs. Although a drug challenge is required for definite confirmation, the patient had used several other NSAIDs with no reaction, obviating the need for further procedures. Finally, even though etoricoxib is a safe drug, a high suspicion index is required in patients with FDE.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Patient's data protectionThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.