The BELIEVE study is a European, non-interventional study which includes patients with overactive bladder who were prescribed mirabegron as part of routine clinical practice. Data from the Spanish subpopulation has been obtained for the present study, aiming to analyze health-related quality-of-life (HRQoL) and treatment persistence of these patients.
Materials and methodsData from 11 Spanish hospitals of the BELIEVE study were analyzed. The primary endpoint was to evaluate change of HRQoL from baseline with overactive bladder questionnaire (OAB-q). Secondary endpoints included treatment persistence, HRQoL based on the EQ-5D-5L questionnaire and adverse events. Study follow-up was 12 months, with two visit windows at 2–4 months and 10–12 months.
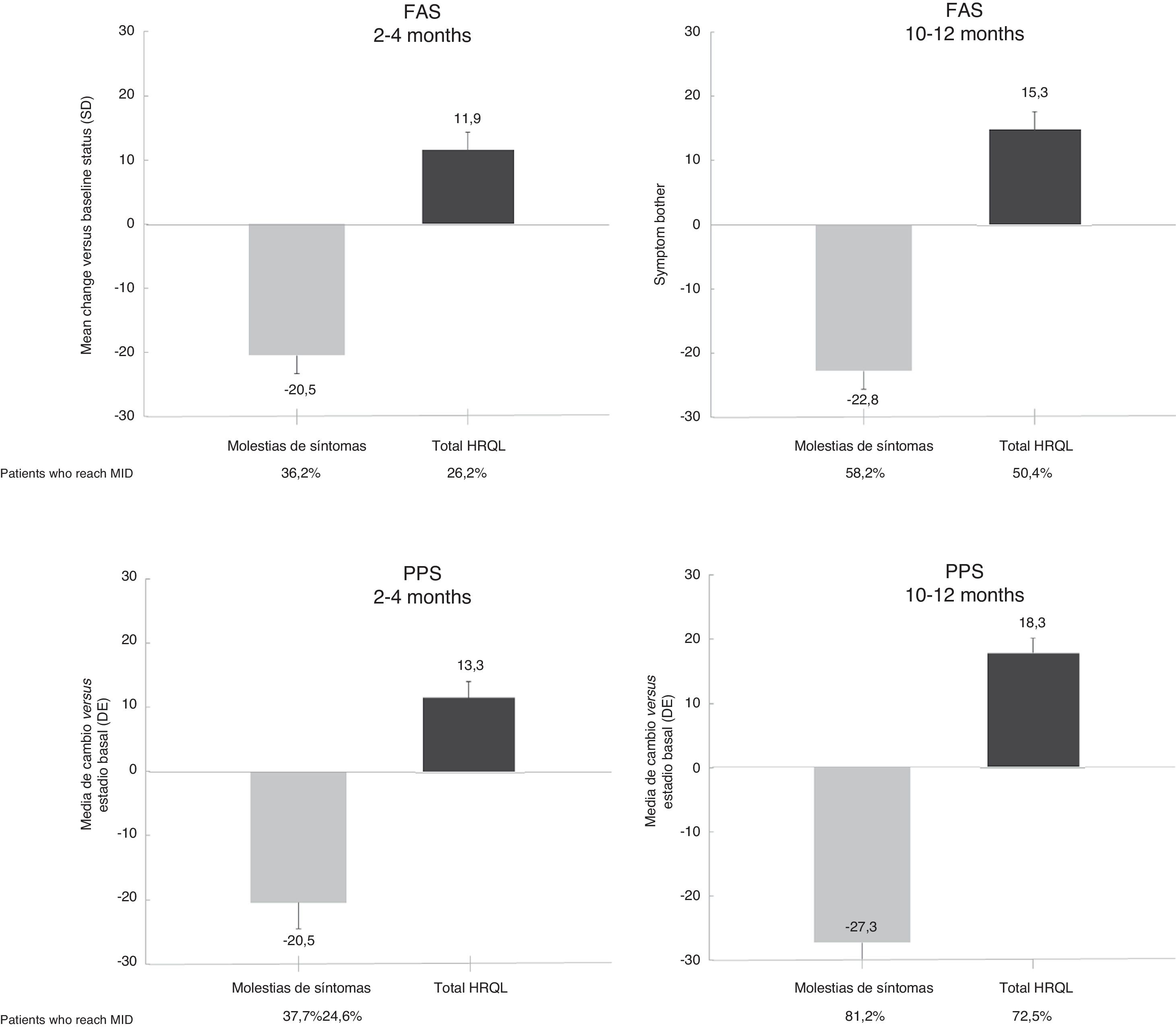
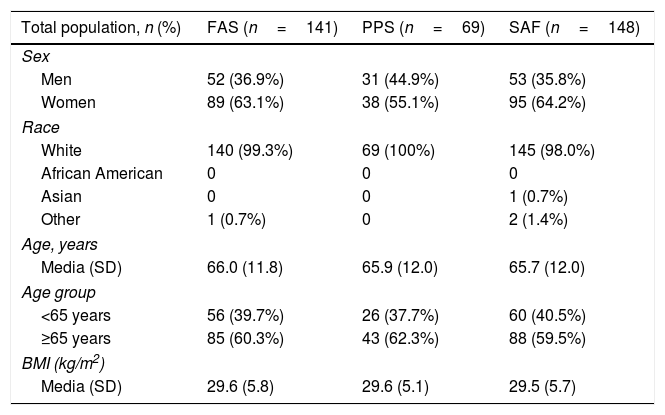
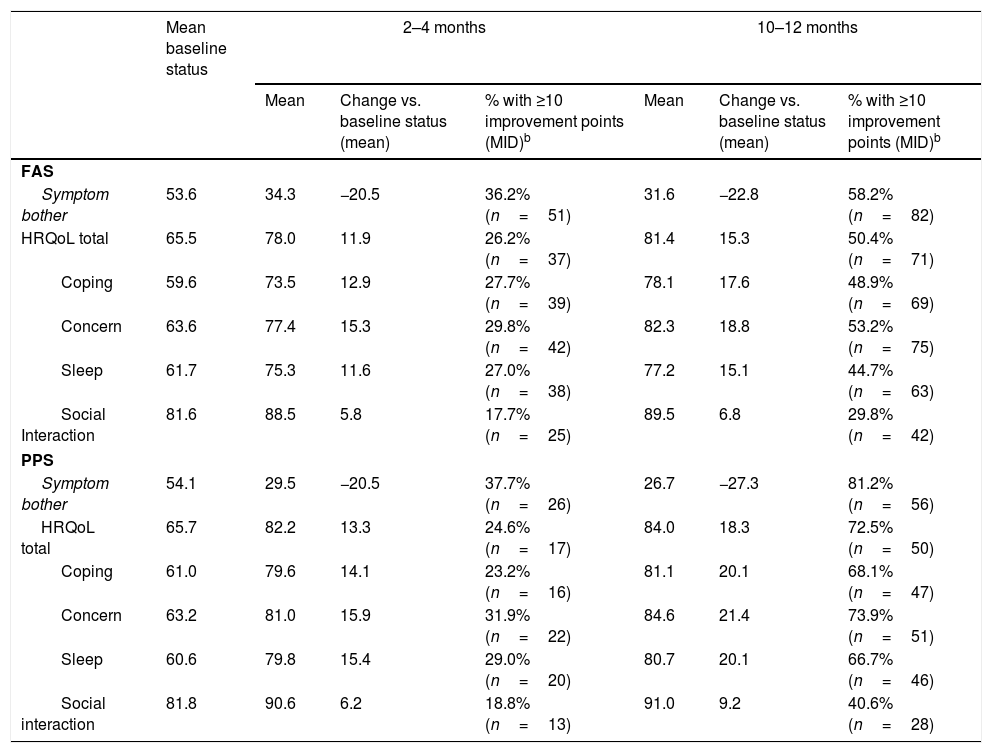
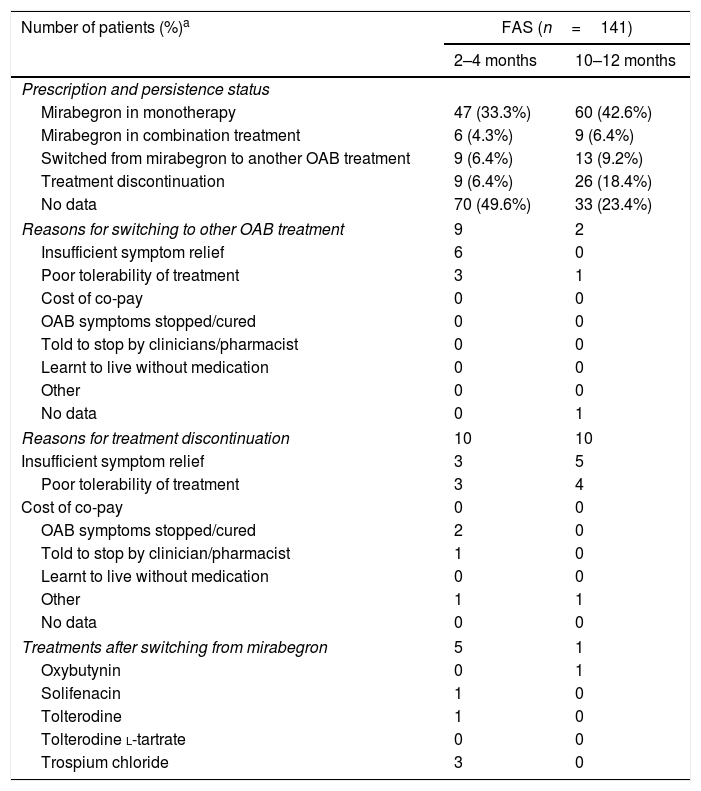
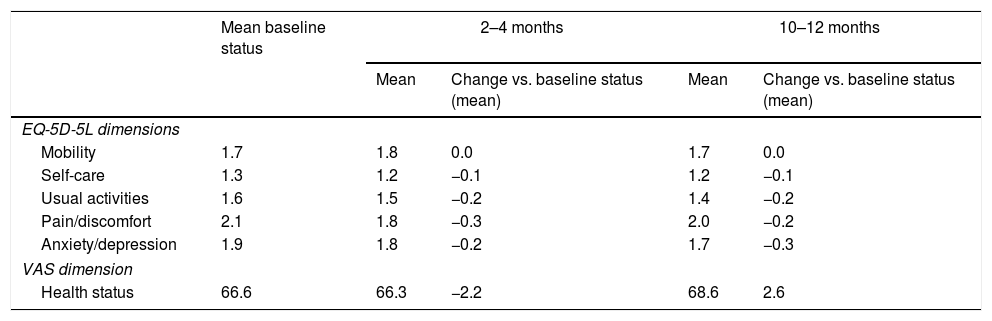
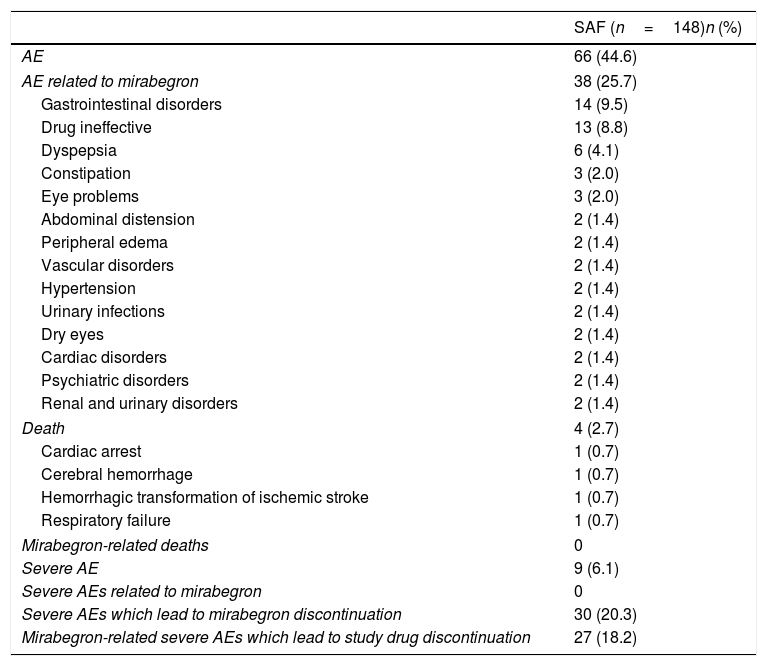
Results153 Spanish patients were enrolled in the study. In the Full Analysis Set (FAS), 63.1% were women, and the mean age was 66 years. Symptom bother and HRQoL improved from baseline to 2–4 months and 10–12 months. EQ-5D-5L questionnaire also showed an improved patients’ HRQoL. Treatment persistence was high, as 49% of patients remained with mirabegron at 10–12 months. Adverse events were consistent with previous safety profile results of mirabegron, and no unexpected safety issues were observed.
ConclusionsSpanish patients treated with mirabegron in real clinical practice reported improvements in HRQoL, with a good tolerability and persistence to treatment.
El estudio BELIEVE es un estudio europeo, no intervencionista, con pacientes con vejiga hiperactiva que recibieron mirabegrón en condiciones de práctica clínica habitual. El presente estudio incluye datos de la subpoblación española, y evalúa su calidad de vida relacionada con la salud (CVRS) y su persistencia al tratamiento.
Materiales y métodosSe analizaron los datos de los 11 centros españoles del estudio BELIEVE. La variable principal fue el cambio de la CVRS desde el estado basal, a partir del cuestionario OAB-q. Las variables secundarias incluyeron la persistencia al tratamiento, la CVRS mediante el EQ-5D-5L y los acontecimientos adversos. El periodo de seguimiento fue de 12 meses, con 2 ventanas a los 2–4 meses y a los 10-12 meses.
ResultadosSe incluyeron 153 pacientes españoles. En el Full Analysis Set (FAS), el 63,1% eran mujeres, con una edad media de 66 años. Las puntuaciones de la molestia de síntomas y la CVRS total mostraron una mejora hasta los 2-4 meses y los 10-12 meses. El EQ-5D-5L también mostró una mejora en la CVRS de estos pacientes. La persistencia fue elevada, ya que el 49% de los pacientes seguían recibiendo mirabegrón a los 10-12 meses. No se observaron problemas de seguridad inesperados y los acontecimientos adversos coincidieron con el perfil de seguridad conocido de mirabegrón.
ConclusionesLos pacientes españoles que reciben mirabegrón en condiciones de práctica clínica real reportan mejoras en su CVRS, con una buena tolerabilidad y una persistencia aceptable al tratamiento.